Direct phenotypic conversion of human fibroblasts into functional osteoblasts triggered by a blockade of the transforming growth factor-β signal
- PMID: 29855543
- PMCID: PMC5981640
- DOI: 10.1038/s41598-018-26745-2
Direct phenotypic conversion of human fibroblasts into functional osteoblasts triggered by a blockade of the transforming growth factor-β signal
Abstract
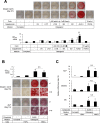
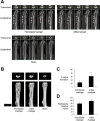
A procedure to generate functional osteoblasts from human somatic cells may pave the way to a novel and effective transplantation therapy in bone disorders. Here, we report that human fibroblasts were induced to show osteoblast phenotypes by culturing with ALK5 i II, which is a specific inhibitor for activin-like kinase 5 (ALK5) (tumor growth factor-β receptor 1 (TGF-β R1)). Cells cultured with ALK5 i II expressed osteoblast-specific genes and massively produced calcified bone matrix, similar to the osteoblasts induced from mesenchymal stem cells (MSC-OBs). Treatment with vitamin D3 in addition to ALK5 i II induced more osteoblast-like characters, and the efficiency of the conversion reached approximately 90%. The chemical compound-mediated directly converted osteoblasts (cOBs) were similar to human primary osteoblasts in terms of expression profiles of osteoblast-related genes. The cOBs abundantly produced bone matrix in vivo and facilitated bone healing after they were transplanted into immunodeficient mice at an artificially induced defect lesion in femoral bone. The present procedure realizes a highly efficient direct conversion of human fibroblasts into transgene-free and highly functional osteoblasts, which might be applied in a novel strategy of bone regeneration therapy in bone diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

