Rapid Push vs Pump-Infused Subcutaneous Immunoglobulin Treatment: a Randomized Crossover Study of Quality of Life in Primary Immunodeficiency Patients
- PMID: 29855752
- PMCID: PMC6028863
- DOI: 10.1007/s10875-018-0507-x
Rapid Push vs Pump-Infused Subcutaneous Immunoglobulin Treatment: a Randomized Crossover Study of Quality of Life in Primary Immunodeficiency Patients
Abstract
Purpose: Subcutaneous immunoglobulin replacement therapy (IgRT) may be administered once a week with a pump or every other day with a syringe (rapid push). The objective of the study was to compare the impact of pump and rapid push infusions on patient's life quality index (LQI).
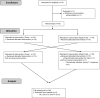
Methods: This study was a randomized, crossover, multicenter, non-inferiority trial conducted in adults with primary immunodeficiency (PID) accustomed to weekly infusions at home by pump. Patients used pump or rapid push for 3 months each according to the randomized sequence. Main criterion was PID-LQI factor I (treatment interference). Non-inferiority ratio was set at 90%.
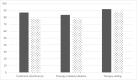
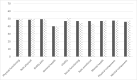
Results: Thirty patients entered the study; 28 completed the two periods. IgRT exposure was similar during each period. At the end of each period, mean LQI factor 1 was 87.0 (IC95% [80.3; 94.3]) and 77.80 (IC95% [71.5; 84.7]) for pump and rapid push, respectively. There was a slightly larger effect of rapid push on treatment interference than with pump so that the primary endpoint could not be met. No difference was found on other LQI components, satisfaction (TSQM), or quality of life (SF36v2). Eight patients declared to prefer rapid push while 19 others preferred pump. Of rapid push infusions, 67.2% led to local reactions vs 71.8% of pump infusions (p = 0.11) illustrating its good tolerance. Rapid push and pump infusions achieved similar trough IgG levels with similar incidence of infections. Rapid push saved 70% of administration cost when compared to pump.
Conclusions: Since IgRT is a lifelong treatment in PID patients, individualization of treatment is of paramount importance. Rapid push is a new administration method in the physician's armamentarium which is preferred by some patients and is cost-effective. CLINICALTRIALS.
Gov identifier: NCT02180763 CLINICAL IMPLICATIONS: Self-administration of small volumes of immunoglobulins at home, every other day, using a syringe (rapid push) is a cost-effective alternative to administration of larger volumes by pump once a week. This study compared subcutaneous infusions of immunoglobulins either weekly via a pump or every other day via a syringe (rapid push). Rapid push is preferred by some patients and is cost-effective, therefore completing a physician's armamentarium.
Keywords: PID immunoglobulin replacement therapy; Primary immunodeficiency; home treatment; rapid push.
Conflict of interest statement
Conflict of Interest
B. Bienvenu, G. Cozon, C. Hoarau, JF. Viallard, and E. Hachulla take part in several scientific boards and/or take part in several studies led by Octapharma. JC. Crave works at Octapharma.
Figures
Similar articles
-
Subcutaneous Gammanorm® by pump or rapid push infusion: Impact of the device on quality of life in adult patients with primary immunodeficiencies.Clin Immunol. 2022 Mar;236:108938. doi: 10.1016/j.clim.2022.108938. Epub 2022 Feb 1. Clin Immunol. 2022. PMID: 35121105 Clinical Trial.
-
Subcutaneous immunoglobulin therapy given by subcutaneous rapid push vs infusion pump: a retrospective analysis.Ann Allergy Asthma Immunol. 2013 Jul;111(1):51-5. doi: 10.1016/j.anai.2013.04.015. Epub 2013 May 22. Ann Allergy Asthma Immunol. 2013. PMID: 23806460
-
Does the route of immunoglobin replacement therapy impact quality of life and satisfaction in patients with primary immunodeficiency? Insights from the French cohort "Visages".Orphanet J Rare Dis. 2016 Jun 22;11(1):83. doi: 10.1186/s13023-016-0452-9. Orphanet J Rare Dis. 2016. PMID: 27334100 Free PMC article.
-
Human immunoglobulin 10 % with recombinant human hyaluronidase: replacement therapy in patients with primary immunodeficiency disorders.BioDrugs. 2014 Aug;28(4):411-20. doi: 10.1007/s40259-014-0104-3. BioDrugs. 2014. PMID: 24925799 Review.
-
Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review.Drugs. 2013 Aug;73(12):1307-19. doi: 10.1007/s40265-013-0094-3. Drugs. 2013. PMID: 23861187 Review.
Cited by
-
Customizing subcutaneous immunoglobulin administration in primary antibody deficiency: patient-centric care perspectives.Immunotherapy. 2024;16(20-22):1235-1245. doi: 10.1080/1750743X.2024.2436343. Epub 2024 Dec 8. Immunotherapy. 2024. PMID: 39648657 Free PMC article. Review.
-
Safety and Tolerability of Subcutaneous IgPro20 at High Infusion Parameters in Patients with Primary Immunodeficiency: Findings from the Pump-Assisted Administration Cohorts of the HILO Study.J Clin Immunol. 2021 Feb;41(2):458-469. doi: 10.1007/s10875-020-00912-5. Epub 2021 Jan 6. J Clin Immunol. 2021. PMID: 33409867 Free PMC article. Clinical Trial.
-
Safety and Tolerability of Manual Push Administration of Subcutaneous IgPro20 at High Infusion Rates in Patients with Primary Immunodeficiency: Findings from the Manual Push Administration Cohort of the HILO Study.J Clin Immunol. 2021 Jan;41(1):66-75. doi: 10.1007/s10875-020-00876-6. Epub 2020 Oct 6. J Clin Immunol. 2021. PMID: 33025378 Free PMC article.
-
A clinician's guide for administration of high-concentration and facilitated subcutaneous immunoglobulin replacement therapy in patients with primary immunodeficiency diseases.Allergy Asthma Clin Immunol. 2022 Sep 30;18(1):87. doi: 10.1186/s13223-022-00726-7. Allergy Asthma Clin Immunol. 2022. PMID: 36180928 Free PMC article. Review.
-
Subcutaneous immunoglobulin treatment for chronic inflammatory demyelinating polyneuropathy.Muscle Nerve. 2021 Sep;64(3):243-254. doi: 10.1002/mus.27356. Epub 2021 Jul 14. Muscle Nerve. 2021. PMID: 34260074 Free PMC article. Review.
References
-
- Al-Herz W, Bousfiha A, Casanova JL, Chapel H, Conley ME, Cunningham-Rundles C, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol. 2011;2:54. doi: 10.3389/fimmu.2011.00054. - DOI - PMC - PubMed
-
- Gardulf A, Bjorvell H, Andersen V, Bjorkander J, Ericson D, Froland SS, Gustafson R, Hammarstrom L, Nystrom T, Soeberg B, Smith CIE. Lifelong treatment with gammaglobulin for primary antibody deficiencies: the patients' experiences of subcutaneous self-infusions and home therapy. J Adv Nurs. 1995;21(5):917–927. doi: 10.1046/j.1365-2648.1995.21050917.x. - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

