Gastric emptying and intestinal appearance of nonabsorbable drugs phenol red and paromomycin in human subjects: A multi-compartment stomach approach
- PMID: 29857136
- PMCID: PMC8883359
- DOI: 10.1016/j.ejpb.2018.05.033
Gastric emptying and intestinal appearance of nonabsorbable drugs phenol red and paromomycin in human subjects: A multi-compartment stomach approach
Abstract
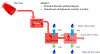
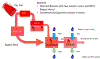
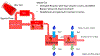
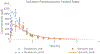
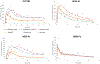
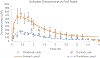
The goal of this study was to create a mass transport model (MTM) model for gastric emptying and upper gastrointestinal (GI) appearance that can capture the in vivo concentration-time profiles of the nonabsorbable drug phenol red in solution in the stomach and upper small intestine by direct luminal measurement while simultaneously recording the contractile activity (motility) via manometry. We advanced from a one-compartmental design of the stomach to a much more appropriate, multi-compartmental 'mixing tank' gastric model that reflects drug distribution along the different regions of the stomach as a consequence of randomly dosing relative to the different contractile phases of the migrating motor complex (MMC). To capture the intraluminal phenol red concentrations in the different segments of the GI tract both in fasted and fed state conditions, it was essential to include a bypass flow compartment ('magenstrasse') to facilitate the transport of the phenol red solution directly to the duodenum (fasted state) or antrum (fed state). The fasted and fed state models were validated with external reference data from an independent aspiration study using another nonabsorbable marker (paromomycin). These results will be essential for the development and optimization of computational programs for GI simulation and absorption prediction, providing a realistic gastric physiologically-based pharmacokinetic (PBPK) model based on direct measurement of gastric concentrations of the drug in the stomach.
Keywords: Aspiration/motility study; Bioavailability; Bioequivalence; Gastrointestinal; Gastrointestinal mass transport model; Human gastric emptying; In vivo dissolution; Nonabsorbable marker; Oral absorption; Paromomycin; Phenol red.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures

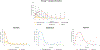
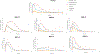

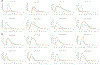
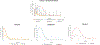
Similar articles
-
In Vivo Dissolution and Systemic Absorption of Immediate Release Ibuprofen in Human Gastrointestinal Tract under Fed and Fasted Conditions.Mol Pharm. 2017 Dec 4;14(12):4295-4304. doi: 10.1021/acs.molpharmaceut.7b00425. Epub 2017 Oct 5. Mol Pharm. 2017. PMID: 28937221 Free PMC article. Clinical Trial.
-
Gastrointestinal transfer: in vivo evaluation and implementation in in vitro and in silico predictive tools.Eur J Pharm Sci. 2014 Oct 15;63:233-42. doi: 10.1016/j.ejps.2014.07.008. Epub 2014 Jul 23. Eur J Pharm Sci. 2014. PMID: 25064697 Clinical Trial.
-
Linking the Gastrointestinal Behavior of Ibuprofen with the Systemic Exposure between and within Humans-Part 2: Fed State.Mol Pharm. 2018 Dec 3;15(12):5468-5478. doi: 10.1021/acs.molpharmaceut.8b00736. Epub 2018 Nov 12. Mol Pharm. 2018. PMID: 30417648 Free PMC article. Clinical Trial.
-
Development of In Vitro Dissolution Testing Methods to Simulate Fed Conditions for Immediate Release Solid Oral Dosage Forms.AAPS J. 2022 Mar 11;24(2):40. doi: 10.1208/s12248-022-00690-5. AAPS J. 2022. PMID: 35277760 Review.
-
Simulating the postprandial stomach: physiological considerations for dissolution and release testing.Mol Pharm. 2013 May 6;10(5):1610-22. doi: 10.1021/mp300604u. Epub 2013 Apr 1. Mol Pharm. 2013. PMID: 23506381 Review.
Cited by
-
Linking the Gastrointestinal Behavior of Ibuprofen with the Systemic Exposure between and within Humans-Part 1: Fasted State Conditions.Mol Pharm. 2018 Dec 3;15(12):5454-5467. doi: 10.1021/acs.molpharmaceut.8b00515. Epub 2018 Nov 12. Mol Pharm. 2018. PMID: 30372084 Free PMC article. Clinical Trial.
-
Measurement of fasted state gastric antral motility before and after a standard bioavailability and bioequivalence 240 mL drink of water: Validation of MRI method against concomitant perfused manometry in healthy participants.PLoS One. 2020 Nov 11;15(11):e0241441. doi: 10.1371/journal.pone.0241441. eCollection 2020. PLoS One. 2020. PMID: 33175860 Free PMC article. Clinical Trial.
-
Stochastic Differential Equation-based Mixed Effects Model of the Fluid Volume in the Fasted Stomach in Healthy Adult Human.AAPS J. 2023 Jul 27;25(5):76. doi: 10.1208/s12248-023-00840-3. AAPS J. 2023. PMID: 37498389
-
Physiologically Based Pharmacokinetic/Pharmacodynamic Modeling to Predict the Impact of CYP2C9 Genetic Polymorphisms, Co-Medication and Formulation on the Pharmacokinetics and Pharmacodynamics of Flurbiprofen.Pharmaceutics. 2020 Nov 2;12(11):1049. doi: 10.3390/pharmaceutics12111049. Pharmaceutics. 2020. PMID: 33147873 Free PMC article.
-
Understanding In Vivo Dissolution of Immediate Release (IR) Solid Oral Drug Products Containing Weak Acid BCS Class 2 (BCS Class 2a) Drugs.AAPS J. 2021 Oct 26;23(6):113. doi: 10.1208/s12248-021-00639-0. AAPS J. 2021. PMID: 34704158
References
-
- Hens B, Tsume Y, Bermejo M, Paixao P, Koenigsknecht MJ, Baker JR, Hasler WL, Lionberger R, Fan J, Dickens J, Shedden K, Wen B, Wysocki J, Loebenberg R, Lee A, Frances A, Amidon G, Yu A, Benninghoff G, Salehi N, Talattof A, Sun D, Amidon GL, Low buffer capacity and alternating motility along the human gastrointestinal tract: implications for in vivo dissolution and absorption of ionizable drugs, Mol. Pharm 14 (2017) 4281–4294, 10.1021/acs.molpharmaceut.7b00426. - DOI - PubMed
-
- Koenigsknecht MJ, Baker JR, Wen B, Frances A, Zhang H, Yu A, Zhao T, Tsume Y, Pai MP, Bleske BE, Zhang X, Lionberger R, Lee A, Amidon GL, Hasler WL, Sun D, In vivo dissolution and systemic absorption of immediate release ibuprofen in human gastrointestinal tract under fed and fasted conditions, Mol. Pharm 14 (2017) 4295–4304, 10.1021/acs.molpharmaceut.7b00425. - DOI - PMC - PubMed
-
- Lennernäs H, Aarons L, Augustijns P, Beato S, Bolger M, Box K, Brewster M, Butler J, Dressman J, Holm R, Julia Frank K, Kendall R, Langguth P, Sydor J, Lindahl A, McAllister M, Muenster U, Müllertz A, Ojala K, Pepin X, Reppas C, Rostami-Hodjegan A, Verwei M, Weitschies W, Wilson C, Karlsson C, Abrahamsson B, Oral biopharmaceutics tools - time for a new initiative - an introduction to the IMI project OrBiTo, Eur. J. Pharm. Sci 57 (2014) 292–299, 10.1016/j.ejps.2013.10.012. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

