3D Bioprinted Artificial Trachea with Epithelial Cells and Chondrogenic-Differentiated Bone Marrow-Derived Mesenchymal Stem Cells
- PMID: 29857483
- PMCID: PMC6032277
- DOI: 10.3390/ijms19061624
3D Bioprinted Artificial Trachea with Epithelial Cells and Chondrogenic-Differentiated Bone Marrow-Derived Mesenchymal Stem Cells
Abstract
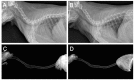
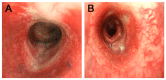
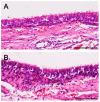
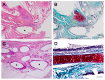
Tracheal resection has limited applicability. Although various tracheal replacement strategies were performed using artificial prosthesis, synthetic stents and tissue transplantation, the best method in tracheal reconstruction remains to be identified. Recent advances in tissue engineering enabled 3D bioprinting using various biocompatible materials including living cells, thereby making the product clinically applicable. Moreover, clinical interest in mesenchymal stem cell has dramatically increased. Here, rabbit bone marrow-derived mesenchymal stem cells (bMSC) and rabbit respiratory epithelial cells were cultured. The chondrogenic differentiation level of bMSC cultured in regular media (MSC) and that in chondrogenic media (d-MSC) were compared. Dual cell-containing artificial trachea were manufactured using a 3D bioprinting method with epithelial cells and undifferentiated bMSC (MSC group, n = 6) or with epithelial cells and chondrogenic-differentiated bMSC (d-MSC group, n = 6). d-MSC showed a relatively higher level of glycosaminoglycan (GAG) accumulation and chondrogenic marker gene expression than MSC in vitro. Neo-epithelialization and neo-vascularization were observed in all groups in vivo but neo-cartilage formation was only noted in d-MSC. The epithelial cells in the 3D bioprinted artificial trachea were effective in respiratory epithelium regeneration. Chondrogenic-differentiated bMSC had more neo-cartilage formation potential in a short period. Nevertheless, the cartilage formation was observed only in a localized area.
Keywords: artificial trachea; bone marrow-derived mesenchymal stem cell; chondrogenic differentiation; three-dimensional bioprinting; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
NR2F2 regulates chondrogenesis of human mesenchymal stem cells in bioprinted cartilage.Biotechnol Bioeng. 2017 Jan;114(1):208-216. doi: 10.1002/bit.26042. Epub 2016 Jul 19. Biotechnol Bioeng. 2017. PMID: 27345768
-
Scaffold-free bioprinted osteogenic and chondrogenic systems to model osteochondral physiology.Biomed Mater. 2019 Oct 3;14(6):065010. doi: 10.1088/1748-605X/ab4243. Biomed Mater. 2019. PMID: 31491773
-
Chondrogenic induction of mesenchymal stromal/stem cells from Wharton's jelly embedded in alginate hydrogel and without added growth factor: an alternative stem cell source for cartilage tissue engineering.Stem Cell Res Ther. 2015 Dec 30;6:260. doi: 10.1186/s13287-015-0263-2. Stem Cell Res Ther. 2015. PMID: 26718750 Free PMC article.
-
Induced pluripotent stem cells in cartilage tissue engineering: a literature review.Biosci Rep. 2024 May 29;44(5):BSR20232102. doi: 10.1042/BSR20232102. Biosci Rep. 2024. PMID: 38563479 Free PMC article. Review.
-
Cartilage 3D bioprinting for rhinoplasty using adipose-derived stem cells as seed cells: Review and recent advances.Cell Prolif. 2023 Apr;56(4):e13417. doi: 10.1111/cpr.13417. Epub 2023 Feb 12. Cell Prolif. 2023. PMID: 36775884 Free PMC article. Review.
Cited by
-
Novel Biomaterials for Tissue Engineering 2018.Int J Mol Sci. 2018 Dec 9;19(12):3960. doi: 10.3390/ijms19123960. Int J Mol Sci. 2018. PMID: 30544860 Free PMC article.
-
Trends in 3D Printing Processes for Biomedical Field: Opportunities and Challenges.J Polym Environ. 2020;28(5):1345-1367. doi: 10.1007/s10924-020-01722-x. Epub 2020 Mar 31. J Polym Environ. 2020. PMID: 32435165 Free PMC article. Review.
-
Cell Culture Model Evolution and Its Impact on Improving Therapy Efficiency in Lung Cancer.Cancers (Basel). 2023 Oct 15;15(20):4996. doi: 10.3390/cancers15204996. Cancers (Basel). 2023. PMID: 37894363 Free PMC article. Review.
-
A defined road to tracheal reconstruction: laser structuring and cell support for rapid clinic translation.Stem Cell Res Ther. 2022 Jul 16;13(1):317. doi: 10.1186/s13287-022-02997-8. Stem Cell Res Ther. 2022. PMID: 35842689 Free PMC article.
-
3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs.Bioengineering (Basel). 2020 Mar 31;7(2):32. doi: 10.3390/bioengineering7020032. Bioengineering (Basel). 2020. PMID: 32244491 Free PMC article. Review.
References
-
- Villegas-Álvarez F., González-Zamora J.F., González-Maciel A., Soriano-Rosales R., Pérez-Guille B., Padilla-Sánchez L., Reynoso-Robles R., Ramos-Morales A., Zenteno-Galindo E., Pérez-Torres A., et al. Fibrocollagen-covered prosthesis for a noncircumferential segmental tracheal replacement. J. Thorac. Cardiovasc. Surg. 2010;139:32–37. doi: 10.1016/j.jtcvs.2009.04.010. - DOI - PubMed
-
- Wurtz A., Hysi I., Kipnis E., Zawadzki C., Hubert T., Jashari R., Copin M.C., Jude B. Tracheal reconstruction with a composite graft: Fascial flap-wrapped allogenic aorta with external cartilage-ring support. Interact. Cardiovasc. Thorac. Surg. 2013;16:37–43. doi: 10.1093/icvts/ivs422. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

