Monocentric Prospective Study into the Sustained Effect of Incobotulinumtoxin A (XEOMIN®) Botulinum Toxin in Chronic Refractory Migraine
- PMID: 29857565
- PMCID: PMC6024863
- DOI: 10.3390/toxins10060221
Monocentric Prospective Study into the Sustained Effect of Incobotulinumtoxin A (XEOMIN®) Botulinum Toxin in Chronic Refractory Migraine
Abstract
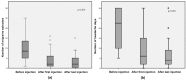
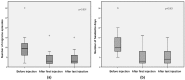
Refractory chronic migraine is a disabling disorder impacting quality of life. BOTOX® (Onabotulinumtoxin A) is approved as a prophylactic treatment of chronic migraine in patients unresponsive to at least three prior preventive treatments. The objective of this study was to determine the prophylactic effect of 145 U XEOMIN® (Incobotulinumtoxin A) injected at 31 specific sites in adult patients with refractory chronic migraine. Sixty-one patients (8 men and 53 women, mean age 50) with migraine were recruited, including 20 patients with isolated chronic migraine, 18 patients with chronic migraine associating tension-type headache, 12 patients with migraine associating medication overuse headache, and 11 patients with episodic disabling migraine. The mean number of injections and duration of treatment per patient was 3.5 (range 2⁻13) and 21 (6⁻68) months, respectively. From baseline to first injection, 44 patients (73%) had >50% reduction in frequency of migraine episodes, 29 patients (48%) showed >50% reduction in number of headache days, and 28 patients (46%) had a >50% reduction in drug intake. Stable response for all three parameters was observed after the last injection. XEOMIN® thus seems to represent an effective and sustained prophylactic treatment of chronic migraine.
Keywords: XEOMIN®; medication overuse headache; prophylactic treatment; refractory chronic migraine; tension headache.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Aurora S.K., Winner P., Freeman M.C., Spierings E.L., Heiring J.O., DeGryse R.E., VanDenburgh A.M., Nolan M.E., Turkel C.C. OnabotulinumtoxinA for Treatment of Chronic Migraine: Pooled Analyses of the 56-Week PREEMPT Clinical Program. Headache. 2011;51:1358–1373. doi: 10.1111/j.1526-4610.2011.01990.x. - DOI - PubMed
-
- Dodick D.W., Turkel C.C., DeGryse R.E., Aurora S.K., Silberstein S.D., Lipton R.B., Diener H.-C., Brin M.F. OnabotulinumtoxinA for Treatment of Chronic Migraine: Pooled Results From the Double-Blind, Randomized, Placebo-Controlled Phases of the PREEMPT Clinical Program. Headache. 2010;50:921–936. doi: 10.1111/j.1526-4610.2010.01678.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

