De Novo Synthesis of Elastin by Exogenous Delivery of Synthetic Modified mRNA into Skin and Elastin-Deficient Cells
- PMID: 29858082
- PMCID: PMC5992474
- DOI: 10.1016/j.omtn.2018.03.013
De Novo Synthesis of Elastin by Exogenous Delivery of Synthetic Modified mRNA into Skin and Elastin-Deficient Cells
Abstract
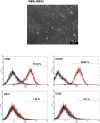
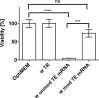
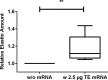
Elastin is one of the most important and abundant extracellular matrix (ECM) proteins that provide elasticity and resilience to tissues and organs, including vascular walls, ligaments, skin, and lung. Besides hereditary diseases, such as Williams-Beuren syndrome (WBS), which results in reduced elastin synthesis, injuries, aging, or acquired diseases can lead to the degradation of existing elastin fibers. Thus, the de novo synthesis of elastin is required in several medical conditions to restore the elasticity of affected tissues. Here, we applied synthetic modified mRNA encoding tropoelastin (TE) for the de novo synthesis of elastin and determined the mRNA-mediated elastin synthesis in cells, as well as ex vivo in porcine skin. EA.hy926 cells, human fibroblasts, and mesenchymal stem cells (MSCs) isolated from a patient with WBS were transfected with 2.5 μg TE mRNA. After 24 hr, the production of elastin was analyzed by Fastin assay and dot blot analyses. Compared with untreated cells, significantly enhanced elastin amounts were detected in TE mRNA transfected cells. The delivered synthetic TE mRNA was even able to significantly increase the elastin production in elastin-deficient MSCs. In porcine skin, approximately 20% higher elastin amount was detected after the intradermal delivery of synthetic mRNA by microinjection. In this study, we demonstrated the successful applicability of synthetic TE encoding mRNA to produce elastin in elastin-deficient cells as well as in skin. Thus, this auspicious mRNA-based integration-free method has a huge potential in the field of regenerative medicine to induce de novo elastin synthesis, e.g., in skin, blood vessels, or alveoli.
Keywords: Williams-Beuren syndrome; elastin; intradermal delivery; skin; synthetic mRNA.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Baldwin A.K., Simpson A., Steer R., Cain S.A., Kielty C.M. Elastic fibres in health and disease. Expert Rev. Mol. Med. 2013;15:e8. - PubMed
-
- Uitto J. Biochemistry of the elastic fibers in normal connective tissues and its alterations in diseases. J. Invest. Dermatol. 1979;72:1–10. - PubMed
-
- Vrhovski B., Weiss A.S. Biochemistry of tropoelastin. Eur. J. Biochem. 1998;258:1–18. - PubMed
-
- Swee M.H., Parks W.C., Pierce R.A. Developmental regulation of elastin production. Expression of tropoelastin pre-mRNA persists after down-regulation of steady-state mRNA levels. J. Biol. Chem. 1995;270:14899–14906. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

