Comparative Effectiveness of DPP-4 Inhibitors Versus Sulfonylurea for the Treatment of Type 2 Diabetes in Routine Clinical Practice: A Retrospective Multicenter Real-World Study
- PMID: 29858976
- PMCID: PMC6064583
- DOI: 10.1007/s13300-018-0452-y
Comparative Effectiveness of DPP-4 Inhibitors Versus Sulfonylurea for the Treatment of Type 2 Diabetes in Routine Clinical Practice: A Retrospective Multicenter Real-World Study
Abstract
Introduction: DPP-4 inhibitors (DPP4i) and sulfonylureas are popular second-line therapies for type 2 diabetes (T2D), but there is a paucity of real-world studies comparing their effectiveness in routine clinical practice.
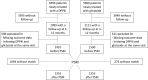
Methods: This was a multicenter retrospective study on diabetes outpatient clinics comparing the effectiveness of DPP4i versus gliclazide extended release. The primary endpoint was change from baseline in HbA1c. Secondary endpoints were changes in fasting plasma glucose, body weight, and systolic blood pressure. Automated software extracted data from the same clinical electronic chart system at all centers. Propensity score matching (PSM) was used to generate comparable cohorts to perform outcome analysis.
Results: We included data on 2410 patients starting DPP4i and 1590 patients starting gliclazide (mainly 30-60 mg/day). At baseline, the two groups differed in disease duration, body weight, blood pressure, HbA1c, fasting glucose, HDL cholesterol, triglycerides, liver enzymes, eGFR, prevalence of microangiopathy, and use of metformin. Among DPP4i molecules, no difference in glycemic effectiveness was detected. In matched cohorts (n = 1316/group), patients starting DPP4i, as compared with patients starting gliclazide, experienced greater reductions in HbA1c (- 0.6% versus - 0.4%; p < 0.001), fasting glucose (- 14.1 mg/dl versus - 8.8 mg/dl; p = 0.007), and body weight (- 0.4 kg versus - 0.1 kg; p = 0.006) after an average 6 months follow-up. DPP4i improved glucose control more than gliclazide, especially in patients who had failed with other glucose-lowering medications or were on basal insulin.
Conclusions: This large retrospective real-world study shows that, in routine clinical practice, starting a DPP4i allows better glycemic control than starting low-dose gliclazide.
Funding: The Italian Diabetes Society, with external support from AstraZeneca.
Keywords: Database; Epidemiology; Pharmacotherapy.
Figures


References
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. - DOI - PubMed
-
- Mishriky BM, Cummings DM, Tanenberg RJ. The efficacy and safety of DPP4 inhibitors compared to sulfonylureas as add-on therapy to metformin in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2015;109:378–388. doi: 10.1016/j.diabres.2015.05.025. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

