Intramuscular versus oral corticosteroids to reduce relapses following discharge from the emergency department for acute asthma
- PMID: 29859017
- PMCID: PMC6513614
- DOI: 10.1002/14651858.CD012629.pub2
Intramuscular versus oral corticosteroids to reduce relapses following discharge from the emergency department for acute asthma
Abstract
Background: Acute asthma is a common cause of presentations to acute care centres, such as the emergency department (ED), and while the majority of patients can be discharged, relapse requiring additional medical care is common. Systemic corticosteroids are a major part in the treatment of moderate to severe acute asthma; however, there is no clear evidence regarding the most effective route of administration for improving outcomes in patients discharged from acute care.
Objectives: To examine the effectiveness and safety of a single dose of intramuscular (IM) corticosteroids provided prior to discharge compared to a short course of oral corticosteroids in the treatment of acute asthma patients discharged from an ED or equivalent acute care setting.
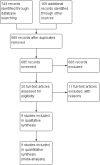
Search methods: The Cochrane Airways Group conducted searches of the Cochrane Airways Group Register of Trials, most recently on 14 March 2018. In addition in April 2017 we completed an extensive search of nine electronic databases including Medline, Embase, EBM ALL, Global Health, International Pharmaceutical Abstracts, CINAHL, SCOPUS, Proquest Dissertations and Theses Global, and LILACS. Furthermore, we searched the grey literature to identify any additional studies.
Selection criteria: We included randomized controlled trials or controlled clinical trials if they compared the effectiveness of intramuscular (IM) versus oral corticosteroids to treat paediatric or adult patients presenting with acute asthma to an ED or equivalent acute care setting. Two independent reviewers assessed study eligibility and study quality. We resolved disagreements via a third party and assessed risk of bias using the Cochrane 'Risk of bias' tool. We assessed the quality of the evidence using GRADE.
Data collection and analysis: For dichotomous outcomes, we calculated individual and pooled statistics as risk ratios (RRs) with 95% confidence intervals (CIs) using a random-effects model. We reported continuous outcomes using mean difference (MD) or standardised mean difference (SMD) with 95% CIs using a random-effects model. We reported heterogeneity using I² and Cochran Q statistics. We used standard procedures recommended by Cochrane.
Main results: Nine studies involving 804 participants (IM = 402 participants; oral = 402 participants) met our review inclusion criteria. Four studies enrolled children (n = 245 participants), while five studies enrolled adults (n = 559 participants). All of the studies recruited participants presenting to an ED, except one study which recruited participants attending a primary care clinic. All of the paediatric studies compared intramuscular (IM) dexamethasone to oral prednisone/prednisolone. In the adult studies, the IM corticosteroid provided ranged from methylprednisolone, betamethasone, dexamethasone, or triamcinolone, while the regimen of oral corticosteroids provided consisted of prednisone, methylprednisolone, or dexamethasone. Only five studies were placebo controlled. For the purposes of this review, we did not take corticosteroid dose equivalency into account in the analysis. The most common co-intervention provided to participants during the acute care visit included short-acting beta₂-agonists (SABA), methylxanthines, and ipratropium bromide. In some instances, some studies reported providing some participants with supplemental oral or IV corticosteroids during their stay in the ED. Co-interventions provided to participants at discharge consisted primarily of SABA, methylxanthine, long-acting beta₂-agonists (LABA), and ipratropium bromide. The risk of bias of the included studies ranged from unclear to high across various domains. The primary outcome of interest was relapse to additional care defined as an unscheduled visit to a health practitioner for worsening asthma symptoms, or requiring subsequent treatment with corticosteroids which may have occurred at any time point after discharge from the ED.We found intramuscular and oral corticosteroids to be similarly effective in reducing the risk for relapse (RR 0.94, 95% CI 0.72 to 1.24; 9 studies, 804 participants; I² = 0%; low-quality evidence). We found no subgroup differences in relapse rates between paediatric and adult participants (P = 0.71), relapse occurring within or after 10 days post-discharge (P = 0.22), or participants with mild/moderate or severe exacerbations (P = 0.35). While we found no statistical difference between participants receiving IM versus oral corticosteroids regarding the risk for adverse events (RR 0.83, 95% CI 0.64 to 1.07; 5 studies, 404 participants; I² = 0%; moderate-quality evidence), an estimated 50 fewer patients per 1000 receiving IM corticosteroids reported experiencing adverse events (95% from 106 fewer to 21 more). We found inconsistent reporting of specific adverse events across the studies. There were no differences in the frequency of specific adverse events including nausea and vomiting, pain, swelling, redness, insomnia, or personality changes. We did not seek additional adverse events data.Participants receiving IM corticosteroids or oral corticosteroids both reported decreases in peak expiratory flow (MD -7.78 L/min, 95% CI -38.83 L/min to 23.28 L/min; 4 studies, 272 participants; I² = 33%; moderate-quality evidence), similar symptom persistence (RR 0.41, 95% CI 0.14 to 1.20; 3 studies, 80 participants; I² = 44%; low-quality evidence), and 24-hour beta-agonist use (RR 0.54, 95% CI 0.21 to 1.37; 2 studies, 48 participants; I² = 0%; low-quality evidence).
Authors' conclusions: There is insufficient evidence to identify whether IM corticosteroids are more effective in reducing relapse compared to oral corticosteroids among children or adults discharged from an ED or equivalent acute care setting for acute asthma. While we found no statistical differences, patients receiving IM corticosteroids reported fewer adverse events. Additional studies comparing the effectiveness of IM versus oral corticosteroids could provide further evidence clarity. Furthermore, there is a need for studies comparing different IM corticosteroids (e.g. IM dexamethasone versus IM methylprednisone) and different oral corticosteroids (e.g. oral dexamethasone versus oral prednisone), with consideration for dosing and pharmacokinetic properties, to better identify the optimal IM or oral corticosteroid regimens to improve patient outcomes. Other factors, such as patient preference and potential issues with adherence, may dictate practitioner prescribing.
Conflict of interest statement
SWK is supported by the Partnerships in Research Innovation in Health Services (PRIHS) Choosing Wisely Project Health. SWK has no known conflicts of interest.
EC is supported by the Department of Emergency Medicine, University of Alberta. EC has no known conflicts of interest.
SC has no known conflicts of interest.
CVR is supported by the Emergency Medicine Research Group (EMeRG). CVR has no known conflicts of interest.
BHR is supported by a Tier I Canada Research Chair in Evidence‐based Emergency Medicine from CIHR through the Government of Canada (Ottawa, Ontario). Several reviews in which he is a co‐author are cited in this review; however, no primary studies from his Emergency Medicine Research Group (EMeRG) were included.
Figures
Update of
References
References to studies included in this review
Al‐Wahadneh 2006 {published data only}
-
- Al‐Wahadneh A, Jboor S, Dahabreh M. A comparison of efficacy and safety of a single dose of intramuscular dexamethasone acetate with oral prednisolone in the management of asthma exacerbations in children. Journal of the Royal Medical Services 2006;13(1):15‐8.
Chan 2001 {published data only}
-
- Chan JS, Cowie RL, Lazarenko GC, Little C, Scott S, Ford GT. Comparison of intramuscular betamethasone and oral prednisone in the prevention of relapse of acute asthma. Canadian Respiratory Journal 2001;8(3):147‐52. [PUBMED: 11420590] - PubMed
Gordon 2007 {published data only}
Gries 2000 {published data only}
Hoffman 1988 {published data only}
-
- Hoffman IB, Fiel SB. Oral versus repository corticosteroid therapy in acute asthma. Chest 1988;93(1):11‐3. [PUBMED: 3275525] - PubMed
Klig 1997 {published data only}
-
- Klig JE, Hodge D, Rutherford MW. Symptomatic improvement following emergency department management of asthma: a pilot study of intramuscular dexamethasone versus oral prednisone. Journal of Asthma 1997;34(5):419‐25. [PUBMED: 9350159] - PubMed
Lahn 2004 {published data only}
Lee 1993 {published data only}
-
- Lee CH, Lee CJ, Lan RS, Tsai YH, Chiang YC, Wang WJ, et al. Repository dexamethasone in the treatment of acute bronchial asthma. Changgeng Yi Xue Za Zhi [Chang Gung Medical Journal] 1993;16(1):25‐9. [PUBMED: 8490772] - PubMed
Schuckman 1998 {published data only}
-
- Schuckman H, DeJulius DP, Blanda M, Gerson LW, DeJulius AJ, Rajaratnam M. Comparison of intramuscular triamcinolone and oral prednisone in the out‐patient treatment of acute asthma: a randomized controlled trial. Annals of Emergency Medicine 1998;31(3):333‐8. [PUBMED: 9506490] - PubMed
References to studies excluded from this review
Andrews 2012 {published data only}
Droszcz 1985 {published data only}
Ducharme 2004 {published data only}
Green 1995 {published data only}
-
- Green SS, Lamb GC, Schmitt S, Kaufman J. Oral versus repository corticosteroid therapy after hospitalization for the treatment of asthma. Journal of Allergy and Clinical Immunology 1995;95(1):15‐22. [PUBMED: 7822659 ] - PubMed
Hofmann 2008 {published data only}
-
- Hofmann M, Rodríguez JE, Klatt C. What is the best treatment for an adult whose asthma exacerbation has not completely responded to 5 days of oral corticosteroids?. Evidence‐Based Practice 2008;11(8):4.
Kelso 2014 {published data only}
Ozpenpe 2011 {published data only}
-
- Ozpenpe O, Aydogan M, Iraneci R. Comparison of the efficacy and side effects of intramuscular, intravenous and oral methylprednisolone used in the acute exacerbation of childhood asthma: A randomized clinical trial. Allergy 2011;66(S94):668. [DOI: 10.1111/j.1398-9995.2011.02650.x] - DOI
Razi 2006 {published data only}
-
- Razi E, Moosavi GA. A comparative efficacy of oral prednisone with intramuscular triamcinolone in acute exacerbation of asthma. Iran Journal of Allergy, Asthma and Immunology 2006;5(1):17‐22. [DOI: ] - PubMed
Watnick 2016 {published data only}
References to studies awaiting assessment
Droszcz 1981 {published data only}
-
- Droszcz W, Lech B, Piotrowska B, Garlicki A. Clinical evaluation of betamethasone depot. Pneumonologia Polska 1981;49(8‐9):645‐8. [PUBMED: 7029485] - PubMed
Additional references
Alangari 2014
Castro‐Rodriguez 2016
CDC 2011
-
- CDC. Asthma in the U.S. CDC Vital Signs. www.cdc.gov/vitalsigns/asthma. May 2011.
Croisant 2014
Ducharme 1993
-
- Ducharme FM, Kramer MS. Relapse following emergency treatment for acute asthma: can it be predicted or prevented?. Journal of Clinical Epidemiology 1993;46(12):1395‐402. [PUBMED: 8263566] - PubMed
Ducharme 2011
-
- Ducharme FM, Zemek RL, Chalut D, McGillivray D, Noya FJ, Resendes S, et al. Written action plan in pediatric emergency room improves asthma prescribing, adherence, and control. American Journal of Respiratory and Critical Care Medicine 2011;183(2):195‐203. [DOI: 10.1164/rccm.201001-0115OC] - DOI - PubMed
Emerman 1999
-
- Emerman CL, Woodruff PG, Cydulka RK, Gibbs MA, Pollack CV Jr, Camargo CA Jr. Prospective multicenter study of relapse following treatment for acute asthma among adults presenting to the emergency department. MARC investigators. Multicenter Asthma Research Collaboration. Chest 1999;115(4):919‐27. [PUBMED: 10208187] - PubMed
Emerman 2001
GINA 2017
-
- Global Initiative for Asthma. Global Initative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. ginasthma.org/2017‐gina‐report‐global‐strategy‐for‐asthma‐management‐and... (accessed 12 September 2016).
GRADEpro GDT [Computer program]
-
- McMaster University (developed by EvidencePrime). GRADEpro GDT. Version accessed 24 November 2016. Hamilton (ON): McMaster University (developed by EvidencePrime), 2015.
Higgins 2011
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Krishnan 2009
Moher 2009
Normansell 2016
Paniagua 2017
-
- Paniagua N, Lopez R, Muñoz N, Tames M, Mojica E, Arana‐Arri E, et al. Randomized trial of dexamethasone versus prednisone for children with acute asthma exacerbations. Journal of Pediatrics 2017;191:190‐6.e1. [DOI: ] - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowe 1999
-
- Rowe BH, Travers AH, Holroyd BR, Kelly KD, Bota GW. Nebulized ipratropium bromide in acute pediatric asthma: does it reduce hospital admissions among children presenting to the emergency department?. Annals of Emergency Medicine 1999;34(1):75‐85. [PUBMED: 10381998] - PubMed
Rowe 2001
Rowe 2007a
Rowe 2007b
-
- Rowe BH, Wong E, Blitz S, Diner B, Mackey D, Ross S, et al. Adding long‐acting beta‐agonists to inhaled corticosteroids after discharge from the emergency department for acute asthma: a randomized controlled trial. Academic Emergency Medicine 2007;14(10):833‐40. [DOI: 10.1197/j.aem.2007.06.020] - DOI - PubMed
Rowe 2009
Rowe 2015
Rowe 2017
-
- Rowe BH, Kirkland SW, Vandermeer B, Campbell S, Newton A, Ducharme FM, et al. Prioritizing systemic corticosteroid treatments to mitigate relapse in adults with acute asthma: A systematic review and network meta‐analysis. Academic Emergency Medicine 2017;24(3):371‐81. [DOI: 10.1111/acem.13107] - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous