A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway
- PMID: 29859019
- PMCID: PMC6057909
- DOI: 10.1111/bph.14383
A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway
Abstract
Background and purpose: Non-alcoholic steatohepatitis (NASH) is the most severe form of non-alcoholic fatty liver disease and is a serious public health problem around the world. There are currently no approved treatments for NASH. FGF21 has recently emerged as a promising drug candidate for metabolic diseases. However, the disadvantages of FGF21 as a clinically useful medicine include its short plasma half-life and poor drug-like properties. Here, we have explored the effects of PsTag600-FGF21, an engineered long-acting FGF21 fusion protein, in mice with NASH and describe some of the underlying mechanisms.
Experimental approach: A long-acting FGF21 was prepared by genetic fusion with a 600 residues polypeptide (PsTag600). We used a choline-deficient high-fat diet-induced model of NASH in mice. The effects on body weight, insulin sensitivity, inflammation and levels of hormones and metabolites were studied first. We further investigated whether PsTag600-FGF21 attenuated inflammation through the Th17-IL17A axis and the associated mechanisms.
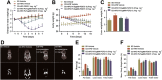
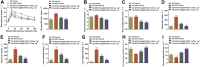
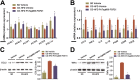
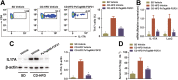
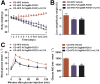
Key results: PsTag600-FGF21 dose-dependently reduced body weight, blood glucose, and insulin and lipid levels and reversed hepatic steatosis. PsTag600-FGF21 enhanced fatty acid activation and mitochondrial β-oxidation in the liver. The profound reduction in hepatic inflammation in NASH mice following PsTag600-FGF21 was associated with inhibition of IL17A expression in Th17 cells. Furthermore, PsTag600-FGF21 depended on adiponectin to exert its suppression of Th17 cell differentiation and IL17A expression.
Conclusions and implications: Our data have uncovered some of the mechanisms by which PsTag600-FGF21 suppresses hepatic inflammation and further suggest that PsTag600-FGF21 could be an effective approach in NASH treatment.
© 2018 The British Pharmacological Society.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

