Selective pharmacological inhibition of DDR1 prevents experimentally-induced glomerulonephritis in prevention and therapeutic regime
- PMID: 29859097
- PMCID: PMC5984769
- DOI: 10.1186/s12967-018-1524-5
Selective pharmacological inhibition of DDR1 prevents experimentally-induced glomerulonephritis in prevention and therapeutic regime
Abstract
Background: Discoidin domain receptor 1 (DDR1) is a collagen-activated receptor tyrosine kinase extensively implicated in diseases such as cancer, atherosclerosis and fibrosis. Multiple preclinical studies, performed using either a gene deletion or a gene silencing approaches, have shown this receptor being a major driver target of fibrosis and glomerulosclerosis.
Methods: The present study investigated the role and relevance of DDR1 in human crescentic glomerulonephritis (GN). Detailed DDR1 expression was first characterized in detail in human GN biopsies using a novel selective anti-DDR1 antibody using immunohistochemistry. Subsequently the protective role of DDR1 was investigated using a highly selective, novel, small molecule inhibitor in a nephrotoxic serum (NTS) GN model in a prophylactic regime and in the NEP25 GN mouse model using a therapeutic intervention regime.
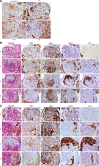
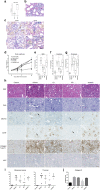
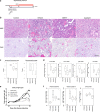
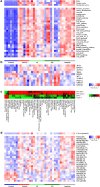
Results: DDR1 expression was shown to be mainly limited to renal epithelium. In humans, DDR1 is highly induced in injured podocytes, in bridging cells expressing both parietal epithelial cell (PEC) and podocyte markers and in a subset of PECs forming the cellular crescents in human GN. Pharmacological inhibition of DDR1 in NTS improved both renal function and histological parameters. These results, obtained using a prophylactic regime, were confirmed in the NEP25 GN mouse model using a therapeutic intervention regime. Gene expression analysis of NTS showed that pharmacological blockade of DDR1 specifically reverted fibrotic and inflammatory gene networks and modulated expression of the glomerular cell gene signature, further validating DDR1 as a major mediator of cell fate in podocytes and PECs.
Conclusions: Together, these results suggest that DDR1 inhibition might be an attractive and promising pharmacological intervention for the treatment of GN, predominantly by targeting the renal epithelium.
Keywords: CKD; DDR1 inhibition; Fibrosis; Glomerulosclerosis.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

