"Exceptional brain aging" without Alzheimer's disease: triggers, accelerators, and the net sum game
- PMID: 29859131
- PMCID: PMC5984828
- DOI: 10.1186/s13195-018-0373-z
"Exceptional brain aging" without Alzheimer's disease: triggers, accelerators, and the net sum game
Abstract
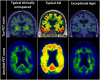
Background: As human longevity increases and Alzheimer's disease (AD) increasingly becomes a significant societal burden, finding pathways or protective factors that facilitate exceptional brain aging without AD pathophysiologies (ADP) will be critical. The goal of this viewpoint is two-fold: 1) to present evidence for "exceptional brain aging" without ADP; and 2) to bring together ideas and observations from the literature and present them as testable hypotheses for biomarker studies to discover protective factors for "exceptional brain aging" without ADP and AD dementia.
Discovering pathways to exceptional aging: There are three testable hypotheses. First, discovering and quantifying links between risk factor(s) and early ADP changes in midlife using longitudinal biomarker studies will be fundamental to understanding why the majority of individuals deviate from normal aging to the AD pathway. Second, a risk factor may have quantifiably greater impact as a trigger and/or accelerator on a specific component of the biomarker cascade (amyloid, tau, neurodegeneration). Finally, and most importantly, while each risk factor may have a different mechanism of action on AD biomarkers, "exceptional aging" and protection against AD dementia will come from "net sum" protection against all components of the biomarker cascade. The knowledge of the mechanism of action of risk factor(s) from hypotheses 1 and 2 will aid in better characterization of their effect on outcomes, identification of subpopulations that would benefit, and the timing at which the risk factor(s) would have the maximal impact. Additionally, hypothesis 3 highlights the importance of multifactorial or multi-domain approaches to "exceptional aging" as well as prevention of AD dementia.
Conclusion: While important strides have been made in identifying risk factors for AD dementia incidence, further efforts are needed to translate these into effective preventive strategies. Using biomarker studies for understanding the mechanism of action, effect size estimation, selection of appropriate end-points, and better subject recruitment based on subpopulation effects are fundamental for better design and success of prevention trials.
Keywords: AD prevention; Biomarker cascade; Exceptional Aging.
Conflict of interest statement
Ethics approval and consent to participate
The data reported here are from Mayo Clinic Study of Aging and from publications by the author. These studies were approved by the Mayo Clinic and Olmsted Medical Center institutional review board. Informed consent was obtained from all participants or their surrogates.
Competing interests
The author declares that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

