Cervical Cancer Incidence in Young U.S. Females After Human Papillomavirus Vaccine Introduction
- PMID: 29859731
- PMCID: PMC6054889
- DOI: 10.1016/j.amepre.2018.03.013
Cervical Cancer Incidence in Young U.S. Females After Human Papillomavirus Vaccine Introduction
Abstract
Introduction: Since 2006, human papillomavirus vaccine has been recommended for young females in the U.S. This study aimed to compare cervical cancer incidence among young women before and after the human papillomavirus vaccine was introduced.
Methods: This cross-sectional study used data from the National Program for Cancer Registries and Surveillance, Epidemiology, and End Results Incidence-U.S. Cancer Statistics 2001-2014 database for U.S. females aged 15-34 years. This study compared the 4-year average annual incidence of invasive cervical cancer in the 4 years before human papillomavirus vaccine was introduced (2003-2006) and the 4 most recent years in the vaccine era (2011-2014). Joinpoint regression models of cervical incidence from 2001 to 2014 were fitted to identify the discrete joints (year) that represent statistically significant changes in the direction of the trend after the introduction of human papillomavirus vaccination in 2006. Data were collected in 2001-2014, released, and analyzed in 2017.
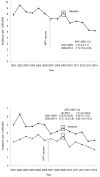
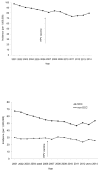
Results: The 4-year average annual incidence rates for cervical cancer in 2011-2014 were 29% lower than that in 2003-2006 (6.0 vs 8.4 per 1,000,000 people, rate ratio=0.71, 95% CI=0.64, 0.80) among females aged 15-24 years, and 13.0% lower among females aged 25-34 years. Joinpoint analyses of cervical cancer incidence among females aged 15-24 years revealed a significant joint at 2009 for both squamous cell carcinoma and non-squamous cell carcinoma. Among females aged 25-34 years, there was no significant decrease in cervical cancer incidence after 2006.
Conclusions: A significant decrease in the incidence of cervical cancer among young females after the introduction of human papillomavirus vaccine may indicate early effects of human papillomavirus vaccination.
Copyright © 2018 American Journal of Preventive Medicine. Published by Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

