Organophosphate pesticide chlorpyrifos impairs STAT1 signaling to induce dopaminergic neurotoxicity: Implications for mitochondria mediated oxidative stress signaling events
- PMID: 29859868
- PMCID: PMC6108448
- DOI: 10.1016/j.nbd.2018.05.019
Organophosphate pesticide chlorpyrifos impairs STAT1 signaling to induce dopaminergic neurotoxicity: Implications for mitochondria mediated oxidative stress signaling events
Abstract
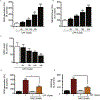
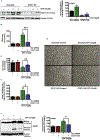
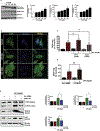
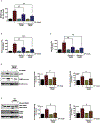
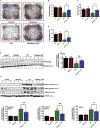
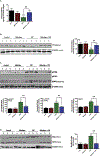
The organophosphate (OP) pesticide chlorpyrifos (CPF), used in agricultural settings, induces developmental and neurological impairments. Recent studies using in vitro cell culture models have reported CPF exposure to have a positive association with mitochondria-mediated oxidative stress response and dopaminergic cell death; however, the mechanism by which mitochondrial reactive oxygen species (ROS) contribute to dopaminergic cell death remains unclear. Therefore, we hypothesized that STAT1, a transcription factor, causes apoptotic dopaminergic cell death via mitochondria-mediated oxidative stress mechanisms. Here we show that exposure of dopaminergic neuronal cells such as N27 cells (immortalized murine mesencephalic dopaminergic cells) to CPF resulted in a dose-dependent increase in apoptotic cell death as measured by MTS assay and DNA fragmentation. Similar effects were observed in CPF-treated human dopaminergic neuronal cells (LUHMES cells), with an associated increase in mitochondrial dysfunction. Moreover, CPF (10 μM) induced time-dependent increase in STAT1 activation coincided with the collapse of mitochondrial transmembrane potential, increase in ROS generation, proteolytic cleavage of protein kinase C delta (PKCδ), inhibition of the mitochondrial basal oxygen consumption rate (OCR), with a concomitant reduction in ATP-linked OCR and reserve capacity, increase in Bax/Bcl-2 ratio and enhancement of autophagy. Additionally, by chromatin immunoprecipitation (ChIP), we demonstrated that STAT1 bound to a putative regulatory sequence in the NOX1 and Bax promoter regions in response to CPF in N27 cells. Interestingly, overexpression of non-phosphorylatable STAT1 mutants (STAT1Y701F and STAT1S727A) but not STAT1 WT construct attenuated the cleavage of PKCδ and ultimately cell death in CPF-treated cells. Furthermore, small interfering RNA knockdown demonstrated STAT1 to be a critical regulator of autophagy and mitochondria-mediated proapoptotic cell signaling events after CPF treatment in N27 cells. Finally, oral administration of CPF (5 mg/kg) in postnatal rats (PNDs 27-61) induced motor deficits, and nigrostriatal dopaminergic neurodegeneration with a concomitant induction of STAT1-dependent proapoptotic cell signaling events. Conversely, co-treatment with mitoapocynin (a mitochondrially-targeted antioxidant) and CPF rescued motor deficits, and restored dopaminergic neuronal survival via abrogation of STAT1-dependent proapoptotic cell signaling events. Taken together, our study identifies a novel mechanism by which STAT1 regulates mitochondria-mediated oxidative stress response, PKCδ activation and autophagy. In this context, the phosphorylation of Tyrosine 701 and Serine 727 in STAT1 was found to be essential for PKCδ cleavage. By attenuating mitochondrial-derived ROS, mitoapocynin may have therapeutic applications for reversing CPF-induced dopaminergic neurotoxicity and associated neurobehavioral deficits as well as neurodegenerative diseases.
Keywords: Autophagy; Chlorpyrifos; Mito-apocynin; Mitochondrial bioenergetics; Neurotoxicity; Oxidative stress; STAT1.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Figures









References
-
- Abolaji AO, et al., 2017. Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem Biol Interact. 270, 15–23. - PubMed
-
- Adams FS, et al., 1996. Characterization and transplantation of two neuronal cell lines with dopaminergic properties. Neurochem Res. 21, 619–27. - PubMed
-
- Ali SF, et al., 1980. Effects of an organophosphate (dichlorvos) on open field behavior and locomotor activity: correlation with regional brain monoamine levels. Psychopharmacology (Berl). 68, 37–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

