TRPCing around the hypothalamus
- PMID: 29859883
- PMCID: PMC6175656
- DOI: 10.1016/j.yfrne.2018.05.004
TRPCing around the hypothalamus
Abstract
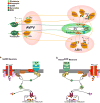
All of the canonical transient receptor potential channels (TRPC) with the exception of TRPC 2 are expressed in hypothalamic neurons and are involved in multiple homeostatic functions. Although the metabotropic glutamate receptors have been shown to be coupled to TRPC channel activation in cortical and sub-cortical brain regions, in the hypothalamus multiple amine and peptidergic G protein-coupled receptors (GPCRs) and growth factor/cytokine receptors are linked to activation of TRPC channels that are vital for reproduction, temperature regulation, arousal and energy homeostasis. In addition to the neurotransmitters, circulating hormones like insulin and leptin through their cognate receptors activate TRPC channels in POMC neurons. Many of the post-synaptic effects of the neurotransmitters and hormones are regulated in different physiological states by expression of TRPC channels in the post-synaptic neurons. Therefore, TRPC channels are key targets not only for neurotransmitters but circulating hormones in their vital role to control multiple hypothalamic functions, which is the focus of this review.
Keywords: 17β-estradiol; GnRH; Insulin; Kisspeptin; Leptin; Neurokinin B; Orexin; POMC; TRPC channels.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have nothing to disclose.
Figures

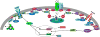
References
-
- Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, Chandarana K, Bell JD, Barsh GS, Smith AJH, Batterham RL, Ashford MLJ, Vanhaesebroeck B, Withers DJ. Dominant role of the p110β isoform of PI3K over p110α in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10:343–354. - PMC - PubMed
-
- Ambudkar IS, Ong HL. Organization and function of TRPC channelsomes. Pflügers Arch. 2007;455:187–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

