GSNOR modulates hyperhomocysteinemia-induced T cell activation and atherosclerosis by switching Akt S-nitrosylation to phosphorylation
- PMID: 29860106
- PMCID: PMC6007174
- DOI: 10.1016/j.redox.2018.04.021
GSNOR modulates hyperhomocysteinemia-induced T cell activation and atherosclerosis by switching Akt S-nitrosylation to phosphorylation
Abstract
The adaptive immune system plays a critical role in hyperhomocysteinemia (HHcy)-accelerated atherosclerosis. Recent studies suggest that HHcy aggravates atherosclerosis with elevated oxidative stress and reduced S-nitrosylation level of redox-sensitive protein residues in the vasculature. However, whether and how S-nitrosylation contributes to T-cell-driven atherosclerosis remain unclear. In the present study, we report that HHcy reduced the level of protein S-nitrosylation in T cells by inducing S-nitrosoglutathione reductase (GSNOR), the key denitrosylase that catalyzes S-nitrosoglutathione (GSNO), which is the main restored form of nitric oxide in vivo. Consequently, secretion of inflammatory cytokines [interferon-γ (IFN-γ) and interleukin-2] and proliferation of T cells were increased. GSNOR knockout or GSNO stimulation rectified HHcy-induced inflammatory cytokine secretion and T-cell proliferation. Site-directed mutagenesis of Akt at Cys224 revealed that S-nitrosylation at this site was pivotal for the reduced phosphorylation at Akt Ser473, which led to impaired Akt signaling. Furthermore, on HHcy challenge, as compared with GSNOR+/+ApoE-/- littermate controls, GSNOR-/-ApoE-/- double knockout mice showed reduced T-cell activation with concurrent reduction of atherosclerosis. Adoptive transfer of GSNOR-/- T cells to ApoE-/- mice fed homocysteine (Hcy) decreased atherosclerosis, with fewer infiltrated T cells and macrophages in plaques. In patients with HHcy and coronary artery disease, the level of plasma Hcy was positively correlated with Gsnor expression in peripheral blood mononuclear cells and IFN-γ+ T cells but inversely correlated with the S-nitrosylation level in T cells. These data reveal that T cells are activated, in part via GSNOR-dependent Akt denitrosylation during HHcy-induced atherosclerosis. Thus, suppression of GSNOR in T cells may reduce the risk of atherosclerosis.
Keywords: Akt; Atherosclerosis; GSNOR; Hyperhomocysteinemia; Medical Biology; Oxidoreductase; Post-Translational Modification; T cell.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

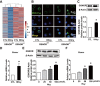

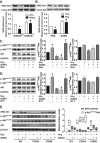
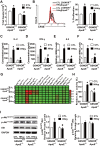

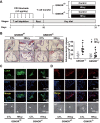

References
-
- Clarke R., Daly L., Robinson K., Naughten E., Cahalane S., Fowler B., Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N. Engl. J. Med. 1991;324:1149–1155. - PubMed
-
- Eikelboom J.W., Lonn E., Genest J., Jr, Hankey G., Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann. Intern Med. 1999;131:363–375. - PubMed
-
- McCully K.S. Homocysteine and vascular disease. Nat. Med. 1996;2:386–389. - PubMed
-
- Dai J., Li W., Chang L., Zhang Z., Tang C., Wang N., Zhu Y., Wang X. Role of redox factor-1 in hyperhomocysteinemia-accelerated atherosclerosis. Free Radic. Biol. Med. 2006;41:1566–1577. - PubMed
-
- Zhang Q., Zeng X., Guo J., Wang X. Oxidant stress mechanism of homocysteine potentiating Con A-induced proliferation in murine splenic T lymphocytes. Cardiovasc Res. 2002;53:1035–1042. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

