Complementary Paths to Chagas Disease Elimination: The Impact of Combining Vector Control With Etiological Treatment
- PMID: 29860294
- PMCID: PMC5982731
- DOI: 10.1093/cid/ciy006
Complementary Paths to Chagas Disease Elimination: The Impact of Combining Vector Control With Etiological Treatment
Abstract
Background: The World Health Organization's 2020 goals for Chagas disease are (1) interrupting vector-borne intradomiciliary transmission and (2) having all infected people under care in endemic countries. Insecticide spraying has proved efficacious for reaching the first goal, but active transmission remains in several regions. For the second, treatment has mostly been restricted to recently infected patients, who comprise only a small proportion of all infected individuals.
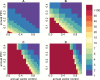
Methods: We extended our previous dynamic transmission model to simulate a domestic Chagas disease transmission cycle and examined the effects of both vector control and etiological treatment on achieving the operational criterion proposed by the Pan American Health Organization for intradomiciliary, vectorial transmission interruption (ie, <2% seroprevalence in children <5 years of age).
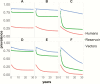
Results: Depending on endemicity, an antivectorial intervention that decreases vector density by 90% annually would achieve the transmission interruption criterion in 2-3 years (low endemicity) to >30 years (high endemicity). When this strategy is combined with annual etiological treatment in 10% of the infected human population, the seroprevalence criterion would be achieved, respectively, in 1 and 11 years.
Conclusions: Combining highly effective vector control with etiological (trypanocidal) treatment in humans would substantially reduce time to transmission interruption as well as infection incidence and prevalence. However, the success of vector control may depend on prevailing vector species. It will be crucial to improve the coverage of screening programs, the performance of diagnostic tests, the proportion of people treated, and the efficacy of trypanocidal drugs. While screening and access can be incremented as part of strengthening the health systems response, improving diagnostics performance and drug efficacy will require further research.
Figures


References
-
- World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 2015; 90:33–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

