Anti-Influenza Hyperimmune Immunoglobulin Enhances Fc-Functional Antibody Immunity During Human Influenza Infection
- PMID: 29860297
- PMCID: PMC6927892
- DOI: 10.1093/infdis/jiy328
Anti-Influenza Hyperimmune Immunoglobulin Enhances Fc-Functional Antibody Immunity During Human Influenza Infection
Abstract
Background: New treatments for severe influenza are needed. Passive transfer of influenza-specific hyperimmune pooled immunoglobulin (Flu-IVIG) boosts neutralizing antibody responses to past strains in influenza-infected subjects. The effect of Flu-IVIG on antibodies with Fc-mediated functions, which may target diverse influenza strains, is unclear.
Methods: We studied the capacity of Flu-IVIG, relative to standard IVIG, to bind to Fcγ receptors and mediate antibody-dependent cellular cytotoxicity in vitro. The effect of Flu-IVIG infusion, compared to placebo infusion, was examined in serial plasma samples from 24 subjects with confirmed influenza infection in the INSIGHT FLU005 pilot study.
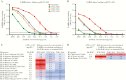
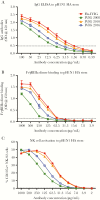
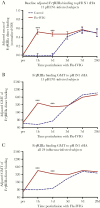
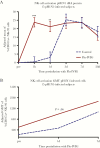
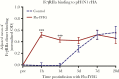
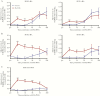
Results: Flu-IVIG contains higher concentrations of Fc-functional antibodies than IVIG against a diverse range of influenza hemagglutinins. Following infusion of Flu-IVIG into influenza-infected subjects, a transient increase in Fc-functional antibodies was present for 1-3 days against infecting and noninfecting strains of influenza.
Conclusions: Flu-IVIG contains antibodies with Fc-mediated functions against influenza virus, and passive transfer of Flu-IVIG increases anti-influenza Fc-functional antibodies in the plasma of influenza-infected subjects. Enhancement of Fc-functional antibodies to a diverse range of influenza strains suggests that Flu-IVIG infusion could prove useful in the context of novel influenza virus infections, when there may be minimal or no neutralizing antibodies in the Flu-IVIG preparation.
Figures

References
-
- World Health Organization. Influenza (seasonal). http://www.who.int/mediacentre/factsheets/fs211/en/. Accessed 5 February 2015.
-
- Hung IFN, To KKW, Lee CK, et al. . Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest 2013; 144:464–73. - PubMed
-
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment?Ann Intern Med 2006; 145:599–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

