Peripapillary Retinoschisis in Glaucoma: Association With Progression and OCT Signs of Müller Cell Involvement
- PMID: 29860466
- PMCID: PMC5983909
- DOI: 10.1167/iovs.18-24160
Peripapillary Retinoschisis in Glaucoma: Association With Progression and OCT Signs of Müller Cell Involvement
Abstract
Purpose: To examine demographic and clinical factors associated with glaucomatous peripapillary retinoschisis (PPRS) and assess its association with glaucoma progression.
Methods: Using a case control study design and longitudinal data from a cohort of 166 subjects with a diagnosis of glaucoma or glaucoma suspect, we compared functional, structural, clinical, and demographic characteristics between PPRS cases and controls.
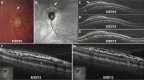
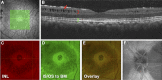
Results: The frequency of PPRS was 6.0% (12 eyes from 10/166 subjects) with two eyes having PPRS in different sectors for a total of 15 retinoschisis events. There were no significant differences (P > 0.05) in age, sex, visual acuity, central corneal thickness, intraocular pressure, or presence of vitreous adhesion between PPRS and controls. However, eyes with PPRS tended to have a higher cup-to-disc ratio (P = 0.06), thinner RNFL (P = 0.02), and worse visual field mean deviation (MD, P = 0.06) than controls. The rate of RNFL thinning was faster in PPRS (average: -2.8%/year; range: -7.4% to 0.0%/year) than controls (-1.3%/year; range: -4.4% to 0.6%/year; P = 0.021). The rate of visual field MD change was faster in PPRS (-0.49 dB/year; range: -2.0 to 0.9 dB/year) than controls (-0.06 dB/year; range: -0.8 to 0.3 dB/year; P = 0.030). OCT scans showed hyperreflective structures spanning the PPRS whose morphology and spacing (50 ± 7 μm) are consistent with Müller glia, causing signal attenuation casting "shadows" onto distal retina.
Conclusions: This is the first report showing that glaucomatous PPRS is associated with a faster overall rate of RNFL thinning and visual field deterioration and to specifically identify OCT signs of Müller cell involvement.
Figures



References
-
- Sauer CG, Gehrig A, Warneke-Wittstock R,et al. . Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat Genet. 1997; 17: 164– 170. - PubMed
-
- Mooy CM, Van Den Born LI, Baarsma S,et al. . Hereditary X-linked juvenile retinoschisis: a review of the role of Müller cells. Arch Ophthalmol. 2002; 120: 979– 984. - PubMed
-
- Byer NE. . Clinical study of senile retinoschisis. Arch Ophthalmol. 1968; 79: 36– 44. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

