Impaired plasticity of macrophages in X-linked adrenoleukodystrophy
- PMID: 29860501
- PMCID: PMC6061697
- DOI: 10.1093/brain/awy127
Impaired plasticity of macrophages in X-linked adrenoleukodystrophy
Abstract
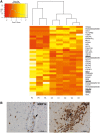
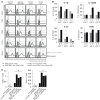
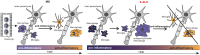
X-linked adrenoleukodystrophy is caused by ATP-binding cassette transporter D1 (ABCD1) mutations and manifests by default as slowly progressive spinal cord axonopathy with associated demyelination (adrenomyloneuropathy). In 60% of male cases, however, X-linked adrenoleukodystrophy converts to devastating cerebral inflammation and demyelination (cerebral adrenoleukodystrophy) with infiltrating blood-derived monocytes and macrophages and cytotoxic T cells that can only be stopped by allogeneic haematopoietic stem cell transplantation or gene therapy at an early stage of the disease. Recently, we identified monocytes/macrophages but not T cells to be severely affected metabolically by ABCD1 deficiency. Here we found by whole transcriptome analysis that, although monocytes of patients with X-linked adrenoleukodystrophy have normal capacity for macrophage differentiation and phagocytosis, they are pro-inflammatory skewed also in patients with adrenomyloneuropathy in the absence of cerebral inflammation. Following lipopolysaccharide activation, the ingestion of myelin debris, normally triggering anti-inflammatory polarization, did not fully reverse the pro-inflammatory status of X-linked adrenoleukodystrophy macrophages. Immunohistochemistry on post-mortem cerebral adrenoleukodystrophy lesions reflected the activation pattern by prominent presence of enlarged lipid-laden macrophages strongly positive for the pro-inflammatory marker co-stimulatory molecule CD86. Comparative analyses of lesions with matching macrophage density in cases of cerebral adrenoleukodystrophy and acute multiple sclerosis showed a similar extent of pro-inflammatory activation but a striking reduction of anti-inflammatory mannose receptor (CD206) and haemoglobin-haptoglobin receptor (CD163) expression on cerebral adrenoleukodystrophy macrophages. Accordingly, ABCD1-deficiency leads to an impaired plasticity of macrophages that is reflected in incomplete establishment of anti-inflammatory responses, thus possibly contributing to the devastating rapidly progressive demyelination in cerebral adrenoleukodystrophy that only in rare cases arrests spontaneously. These findings emphasize monocytes/macrophages as crucial therapeutic targets for preventing or stopping myelin destruction in patients with X-linked adrenoleukodystrophy.
Figures



References
-
- Boven LA, Van Meurs M, Van Zwam M, Wierenga-Wolf A, Hintzen RQ, Boot RG et al. . Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 2006; 129 (Pt 2): 517–26. - PubMed
-
- Brück W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA et al. . Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 1995; 38: 788–96. - PubMed
-
- Budka H, Sluga E, Heiss WD. Spastic paraplegia associated with Addison's disease: adult variant of adreno-leukodystrophy. J Neurol 1976; 213: 237–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

