Continuous versus intermittent antibiotics for bronchiectasis
- PMID: 29860722
- PMCID: PMC6513232
- DOI: 10.1002/14651858.CD012733.pub2
Continuous versus intermittent antibiotics for bronchiectasis
Abstract
Background: Bronchiectasis is a chronic airway disease characterised by a destructive cycle of recurrent airway infection, inflammation and tissue damage. Antibiotics are a main treatment for bronchiectasis. The aim of continuous therapy with prophylactic antibiotics is to suppress bacterial load, but bacteria may become resistant to the antibiotic, leading to a loss of effectiveness. On the other hand, intermittent prophylactic antibiotics, given over a predefined duration and interval, may reduce antibiotic selection pressure and reduce or prevent the development of resistance. This systematic review aimed to evaluate the current evidence for studies comparing continuous versus intermittent administration of antibiotic treatment in bronchiectasis in terms of clinical efficacy, the emergence of resistance and serious adverse events.
Objectives: To evaluate the effectiveness of continuous versus intermittent antibiotics in the treatment of adults and children with bronchiectasis, using the primary outcomes of exacerbations, antibiotic resistance and serious adverse events.
Search methods: On 1 August 2017 and 4 May 2018 we searched the Cochrane Airways Review Group Specialised Register (CAGR), CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, and AMED. On 25 September 2017 and 4 May 2018 we also searched www.clinicaltrials.gov, the World Health Organization (WHO) trials portal, conference proceedings and the reference lists of existing systematic reviews.
Selection criteria: We planned to include randomised controlled trials (RCTs) of adults or children with bronchiectasis that compared continuous versus intermittent administration of long-term prophylactic antibiotics of at least three months' duration. We considered eligible studies reported as full-text articles, as abstracts only and unpublished data.
Data collection and analysis: Two review authors independently screened the search results and full-text reports.
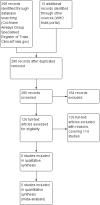
Main results: We identified 268 unique records. Of these we retrieved and examined 126 full-text reports, representing 114 studies, but none of these studies met our inclusion criteria.
Authors' conclusions: No randomised controlled trials have compared the effectiveness and risks of continuous antibiotic therapy versus intermittent antibiotic therapy for bronchiectasis. High-quality clinical trials are needed to establish which of these interventions is more effective for reducing the frequency and duration of exacerbations, antibiotic resistance and the occurrence of serious adverse events.
Conflict of interest statement
J Chalmers: part of the EMBARC group that set research priorities in bronchiectasis. He also receives grant support from Pfizer, AstraZeneca and GlaxoSmithKline. In addition, he is part of an innovative medicines initiative consortium that includes Novartis and Basilea Pharmaceutica. He has participated in advisory boards for Bayer HealthCare, Chiesi and Raptor Pharmaceuticals. He has received fees for speaking from Napp, AstraZeneca, BI and Pfizer. None of these conflicts of interest are related to the work of this review and are unrelated to the topic of the review.
T Donovan: none known.
L Felix: none known.
A Mathioudakis: none known.
S Milan: none known.
S Spencer: named co‐investigator on a study conducted to develop a series of reviews on bronchiectasis.
Update of
References
References to studies excluded from this review
Aksamit 2016 {published data only}
-
- Aksamit TR, Soyza A, Operschall E, Bandel T‐J, Krahn U, Montegriffo E, et al. Baseline demographic profile of subjects of the phase 3 RESPIRE 2 trial of ciprofloxacin dry powder for inhalation (DPI) in non‐cystic fibrosis bronchiectasis (NCFB). American Journal of Respiratory and Critical Care Medicine 2016;193:A1768.
Aksamit 2017 {published data only}
-
- Aksamit T, Bandel TJ, Criollo M, Soyza A, Elborn JS, Operschall E, et al. The RESPIRE trials: two phase III, randomized, multicentre, placebo‐controlled trials of ciprofloxacin dry powder for inhalation (ciprofloxacin dpi) in non‐cystic fibrosis bronchiectasis. Contemporary Clinical Trials 2017; Vol. 58:78‐85. - PubMed
-
- Aksamit TR, Bandel T‐J, Criollo M, Elborn JS, Lau M, Operschall E, et al. Respire 2: ciprofloxacin dpi 32.5 mg b.i.d. administered 14 days on/off or 28 days on/off vs. placebo for 48 weeks in patients with non‐cystic fibrosis bronchiectasis (NCFB). American Journal of Respiratory and Critical Care Medicine 2017;195:A7642.
-
- Soyza A, Aksamit T, Operschall E, Bandel T‐J, Krahn U, Criollo M, et al. Baseline demographic profile of subjects of the phase 3 RESPIRE 1 trial of ciprofloxacin dry powder for inhalation (DPI) in non‐cystic fibrosis bronchiectasis (NCFB). European Respiratory Journal 2015;46:PA2617.
-
- Soyza A, Aksamit TR, Bandel T‐J, Criollo M, Elborn JS, Krahn U, et al. RESPIRE 1: ciprofloxacin DPI 32.5mg b.d. administered 14 day on/off or 28 day on/off vs placebo for 48 weeks in subjects with non‐cystic fibrosis bronchiectasis (NCFB). European Respiratory Journal 2016;48:OA272.
-
- NCT01764841. Ciprofloxacin dry powder for inhalation in non‐cystic fibrosis bronchiectasis (non‐cf BE) [Randomized, double‐blind, placebo‐controlled, multicenter study comparing ciprofloxacin dpi 32.5 mg bid (twice a day) intermittently administered for 28 days on / 28 days off or 14 days on / 14 days off versus placebo to evaluate the time to first pulmonary exacerbation and frequency of exacerbations in subjects with non‐cystic fibrosis bronchiectasis]. clinicaltrials.gov/ct2/show/NCT01764841 (first received 10 January 2013).
Alberto 1968 {published data only}
-
- Alberto S, Colongo PG, Brusasco L, Frigerio G. Studies of the clinical and respiratory functional effects of a mucolytic‐antibiotic preparation in chronic bronchopulmonary diseases. Controlled double‐blind single code studies. Minerva Medica 1968;59(53):2995‐3002. - PubMed
Alekseenko 1978 {published data only}
-
- Alekseenko AV, Sokolov SB, Kobrin LI, Opalinskii VN, Lavrent'ev IuA. Direct current in the overall treatment of inflammatory suppurative diseases of the lungs [Voprosy Kurortologii, Fizioterapii, i Lechebonoi]. Fizicheskoi Kultury 1978;2:44‐6. - PubMed
Alekseenko 1980 {published data only}
-
- Alekseenko AV. Effectiveness of conservative treatment of disseminated forms of chronic inflammatory‐suppurative lung diseases. Terapevticheskii Arkhiv 1980;52(1):57‐9. - PubMed
Aliberti 2016 {published data only}
-
- Aliberti S, Masefield S, Polverino E, Soyza A, Loebinger MR, Menendez R, et al. Research priorities in bronchiectasis: a consensus statement from the EMBARC Clinical Research Collaboration. European Respiratory Journal 2016;48(3):632‐47. [PUBMED: 27288031] - PubMed
Al‐Mobeireek 1998 {published data only}
-
- Al‐Mobeireek A, Kambal AM, Al‐Balla SR, Al‐Sawwaf H, Saleemi S. Pseudomonas aeruginosa in hospitalized patients with infective exacerbations of bronchiectasis: clinical and research implications. Annals of Saudi Medicine 1998;18(5):469‐71. - PubMed
Antoniu 2013 {published data only}
-
- Antoniu S, Azoicai D. Ciprofloxacin dpi in non‐cystic fibrosis bronchiectasis: a phase II randomized study. Expert Opinion on Investigational Drugs 2013;22(5):671‐3. - PubMed
Cherniack 1959 {published data only}
-
- Cherniack NS, Vosti KL, Dowling HF, Lepper MH, Jackson GG. Long‐term treatment of bronchiectasis and chronic bronchitis; a controlled study of the effects of tetracycline, penicillin, and an oleandomycinpenicillin mixture. Archives of Internal Medicine 1959;103(3):345‐53. - PubMed
ChiCTR‐IOR‐16008910 2016 {published data only}
-
- ChiCTR‐IOR‐16008910. Effectiveness of procalcitonin‐guided antibiotic therapy in acute exacerbations of bronchiectasis: a randomized controlled trial. www.chictr.org.cn/showprojen.aspx?proj=15079 (first received 26 July 2016).
Chonabayasi 1988 {published data only}
-
- Chonabayasi N, Aosima M, Noguchi M, Yoshimura K, Nakatani T, Nakamori Y, et al. T‐3262 in respiratory infections. Chemotherapy 1988; Vol. 36:439‐53.
Choo 2018 {published data only}
Cseri 1975 {published data only}
-
- Cseri T, Kelemen S. Treatment with sumetrolim in chronic non‐specific respiratory tract infections. Therapia Hungarica 1975;23(3):115‐7. - PubMed
Currie 1987 {published data only}
-
- Currie DC, Garbett ND, Chan KL, Higgs E, Todd H, Nunn AJ, et al. Double blind randomised placebo controlled study of long term high dose antibiotic in patients with bronchiectasis [abstract]. Clinical Research 1987;72(s16):79P.
Dimakou 2017 {published data only}
-
- Dimakou K, Kaponi M, Karampitsakos T, Tzouvelekis A, Chrysikos S, Melachroinidou M, et al. Eradication treatment in Non CF bronchiectasis: the effect of inhaled antibiotics (tobramycin and colistin) on patients with pseudomonas aeruginosa. European Respiratory Journal 2017;suppl 61:OA1971.
-
- Dimakou K, Liapikou A, Triantafilidou C, Chrysikos S, Kaponi M, Melachroinidou M, et al. Non cf bronchiectasis: the effect of inhaled antibiotics (tobramycin and colistin) in patients with pseudomonas aeruginosa in sputum. American Journal of Respiratory and Critical Care Medicine 2017;195:A1518.
Douglas 1957 {published data only}
-
- Douglas AC, Somner AR, Marks BL, Grant IWB. Effect of antibiotics on purulent sputum in chronic bronchitis and bronchiectasis. Lancet 1957;273(6988):214‐8. - PubMed
Hayashi 1989 {published data only}
-
- Hayashi T, Yamada H, Yasuoka A, Sasayama K, Doutsu Y, Kohno S, et al. Basic and clinical studies on cefdinir. Chemotherapy 1989;37(Supplement 2):565‐78.
Hill 1992 {published data only}
-
- Hill SL, Bilton D, Johnson MM, Pye A, Mitchell JL, Stockley RA. Sputum and serum pharmacokinetics of loracarbef (a new carbacephem antibiotic) in patients with bronchiectasis [Abstract]. European Respiratory Journal 1992;5:142s.
Howie 1976 {published data only}
Huang 2018 {published data only}
Hughes 1973 {published data only}
-
- Hughes DT. Use of combinations of trimethoprim and sulfamethoxazole in the treatment of chest infections. Journal of Infectious Diseases 1973;128(Suppl):701‐5. - PubMed
-
- Hughes DT. Use of combinations of trimethoprim and sulphamethoxazole in the treatment of chest infections. Medical Journal of Australia 1973;1(2):58‐61. - PubMed
Iglauer 1973 {published data only}
-
- Iglauer E, Wieser O. Parenteral treatment of severe bronchopulmonary infections using trimethoprim and sulfamethoxazole. Wiener Medizinische Wochenschrift (1946) 1973; Vol. 123, issue 20:322‐3. - PubMed
Inoue 1989 {published data only}
-
- Inoue Y, Masuyama Y, Dotsu Y, Kaku M, Suyama N, Hayashi T, et al. Basic and clinical studies on 7432‐S. [Japanese]. Chemotherapy 1989;37:285‐97.
Irabu 1989 {published data only}
-
- Irabu Y, Tamaki K, Fukuhara H, Nakamura H, Kaneshima H, Shimoij K, et al. Laboratory and clinical studies on cefdinir. [Japanese]. Chemotherapy 1989;37:603‐11.
Irabu 1992 {published data only}
-
- Irabu Y, Fukuhara H, Nakamura H, Kaneshima H, Simoji K, Kitsukawa K, et al. Laboratory and clinical studies of ME1207. [Japanese]. Chemotherapy 1992;40:452‐8.
Juthong 2011 {published data only}
-
- Juthong S, Eiamsa‐ard S. The effects of roxithromycin as anti‐inflammatory agent on clinical outcomes in patient with bronchiectasis: A double blinded randomized controlled study [Abstract]. European Respiratory Journal 2011;38:819s.
Kanai 1963 {published data only}
-
- Kanai J, Tanaka S, Matsumoto T, Noda S. Clinical trial use of phenoxypropyl penicillin. Journal of Antibiotics 1963;16:25‐6. - PubMed
Kawano 1974 {published data only}
-
- Kawano M, Irimajiri K, Higuchi J, Uchiyama T, Tsubura E. Studies on amikacin (BB‐K8), a new aminoglycoside antibiotic (author's transl). Japanese Journal of Antibiotics 1974;27(4):446‐50. - PubMed
Kawashima 1989 {published data only}
-
- Kawashima K, Yamamoto H, Kadoya M, Kato M, Nata T, Iida E, et al. Clinical role of Branhamella catarrhalis infection in pulmonary disorders. [Japanese]. Saishin Igaku [Modern Medicine] 1989;44(6):1268‐72.
Koyama 1992 {published data only}
-
- Koyama M. ME1207: a clinical study. [Japanese]. Chemotherapy 1992;40:692‐5.
Krawczyk 1981 {published data only}
-
- Krawczyk Z, Gornicka Wilczynska M, Pilarska Machowicz A, Pruszynska S. Comparative evaluation of certain antibiotics and Biceptol preparation in the treatment of chronic bronchial disease exacerbations. [Polish]. Polski Tygodnik Lekarski 1981;36(25):927‐30. - PubMed
Kudo 1992 {published data only}
-
- Kudo K, Ishii A, Kabe J. ME1207 in respiratory infections. [Japanese]. Chemotherapy 1992;40:696‐9.
Kurishima 1992 {published data only}
-
- Kurishima S, Hirose H, Fukui T, Katsu M. Basic and clinical studies on cefclidin. [Japanese]. Chemotherapy 1992;40:273‐9.
Kuze 1988 {published data only}
-
- Kuze F, Kurasawa T, Kato M, Kagioka A, Inaba N, Kuroda N, et al. Cefodizime (THR‐221) in respiratory tract infections. [Japanese]. Chemotherapy 1988;36:467‐72.
Lamotte 1981 {published data only}
-
- Lamotte A. Bronchiectasis [French]. Ouest‐medical 1981;34(14):969‐72.
Ledson 2000 {published data only}
Lunacharskaia 1968 {published data only}
-
- Lunacharskaia TV, Kopeiko IP. Intratracheal use of morphocycline. [Russian]. Antibiotiki 1968;13(12):1123‐6. - PubMed
Maekawa 1967 {published data only}
-
- Maekawa N, Tsukuma S, Nakanishi M, Yamashita M, Kanda M. Clinical results of the use of cephaloridine in lung diseases, especially pulmonary tuberculosis. [Japanese]. Journal of Antibiotics. Ser. B 1967;20(2):140‐4. - PubMed
Masuno 1992 {published data only}
-
- Masuno T, Ikeda T, Kishimoto S, Morimoto Y, Tsujimoto T, Igarashi T, et al. A clinical study of cefclidin. Chemotherapy 1992;40:331‐40.
Matsumoto 1984 {published data only}
-
- Matsumoto K, Nagatake T, Shishido H. Laboratory and clinical evaluation of lenampicillin (KBT‐1585) in respiratory infection. [Japanese]. Chemotherapy 1984;32:397‐411.
Matsumoto 1992 {published data only}
-
- Matsumoto M, Koga Y, Ando M, Araki S. Clinical studies on ME1207 in the treatment of respiratory tract infections. [Japanese]. Chemotherapy 1992;40:740‐3.
Matsuura 1993 {published data only}
-
- Matsuura T, Suzuki K, Yamamoto T. Clinical studies of S‐1108 in elderly patients. Chemotherapy 1993;41:795‐9.
May 1974 {published data only}
-
- May JR, Ingold H. Amoxicillin in the treatment of lower respiratory infections. Advances in Clinical Pharmacology 1974;7:85‐90. - PubMed
Mazzei 1993 {published data only}
-
- Mazzei JA, Vujacich P, Fernandez A, Tanco J. Ciprofloxacin in the treatment of lower respiratory tract infections. Drugs 1993;46:407‐9.
Messens 1973 {published data only}
-
- Messens Y, Oger A, Cornette M, Calay G. Double‐blind study of the sulfamethoxazole‐trimethoprim association in the treatment of pulmonary infections. Acta Clinica Belgica 1973;28(2):92‐9. - PubMed
Milcev 1985 {published data only}
-
- Milcev M, Ovcarova H, Ivanov S. Antibacterial treatment of anaerobic lung infections. [Bulgarian]. Pnevmologiya i Ftiziatriya 1985;22(3):20‐4.
Ming 2005 {published data only}
-
- Ming O, Yong Li, Zhang W. Efficacy of macrolide and theophylline in the management of bronchiectasis. Respirology 2005;10:A168.
Molodtsova 1980 {published data only}
-
- Molodtsova VP, Fedoseev GB, Gerasin VA. Cleansing of the bronchial tree in treating chronic inflammatory lung diseases. [Russian]. Sovetskaia Meditsina 1980;11(11):90‐2. - PubMed
Morrone 1989 {published data only}
-
- Morrone N, Saito M, Brito VC, Hayashi F. Pulmonary infections in adults: two therapeutics regimens of ofloxacin. Folha Medica 1989;98(4):253‐8.
Murayama 1992 {published data only}
-
- Murayama T, Kuze F, Oida K, Nanbu Y, Iwata T, Ikeda N, et al. A clinical study of the effects of ME1207 on respiratory infections [Japanese]. Chemotherapy 1992;40:724‐7.
Nagy 1968 {published data only}
-
- Nagy M, Meszaros G. Late results of the conservative treatment of bronchiectasis in adults. [German]. Zeitschrift fur Tuberkulose und Erkrankungen der Thoraxorgane 1968;127(5):283‐90. - PubMed
Nakagawa 1989 {published data only}
-
- Nakagawa Y, Niki Y, Hino J, Kishimoto T, Kawane H, Soejima R. Bacteriological and clinical studies of cefdinir. Chemotherapy 1989; Vol. 37:518‐24.
Nakagawa 1993 {published data only}
-
- Nakagawa M, Tsubura E. Clinical study of S‐1108 in respiratory infections. Chemotherapy 1993;41:803‐6.
Nasu 1988 {published data only}
-
- Nasu M, Yamazaki T, Yamazaki H, Kuroda Y, Goto Y, Shigeno H, et al. T‐3262 in respiratory tract infections [Japanese]. Chemotherapy 1988;36:699‐709.
NCT00415350 {published data only}
-
- NCT00415350. Bronchiectasis and long term azithromycin treatment [Bronchiectasis and long term azithromycin treatment: a randomised placebo‐controlled trial studying disease modifying effects of immunomodulating treatment]. clinicaltrials.gov/ct2/show/NCT00415350 (first received 22 December 2006).
NCT00524095 {published data only}
-
- NCT00524095. Bronchiectasis in chronic obstructive pulmonary disease (COPD) patients: role of prophylaxis [Bronchiectasis in COPD patients : role of prophylaxis with inhaled steroids and antibiotic on the natural history of the disease]. clinicaltrials.gov/ct2/show/NCT00524095 (first received 3 September 2007).
NCT00775138 {published data only}
-
- NCT00775138. A study to determine the safety and tolerability of arikace versus placebo in patients who have bronchiectasis [A placebo controlled, randomized, parallel cohort, safety and tolerability study of 2 dose levels of liposomal amikacin for inhalation (Arikace™) in patients with bronchiectasis complicated by chronic infection due to pseudomonas aeruginosa]. clinicaltrials.gov/ct2/show/NCT00775138 (first received 17 October 2008).
NCT00889967 {published data only}
-
- NCT00889967. Safety and efficacy study of ciprofloxacin for inhalation in patients with non‐cystic fibrosis bronchiectasis "ORBIT‐1" [An international, multicenter, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy, safety, and tolerability of once daily administration of two strengths of ciprofloxacin for inhalation compared with placebo for inhalation in the management of pseudomonas aeruginosa in patients with non cystic fibrosis bronchiectasis]. clinicaltrials.gov/ct2/show/NCT00889967 (first received 29 April 2009).
NCT00930982 {published data only}
-
- NCT00930982. Evaluation of cipro inhale in patients with non‐cystic fibrosis bronchiectasis [Randomized, placebo‐controlled, double‐blind, multi‐center study to evaluate the safety and efficacy of ciprofloxacin inhale compared to placebo in patients with non‐cystic fibrosis bronchiectasis]. clinicaltrials.gov/ct2/show/NCT00930982 (first received 2 July 2009).
NCT01313624 {published data only}
-
- NCT01313624. A study to see if AZLI (an inhaled antibiotic) is effective in treating adults with non‐cf bronchiectasis ‐ AIR‐BX1 [A phase 3, double‐blind, multicenter, randomized, placebo‐controlled trial evaluating repeated courses of aztreonam for inhalation solution in subjects with non‐cf bronchiectasis and gram‐negative endobronchial infection]. clinicaltrials.gov/ct2/show/NCT01313624 (first received 14 March 2011).
NCT01515007 {published data only}
-
- NCT01515007. Phase 3 Study With Ciprofloxacin Dispersion for Inhalation in Non‐CF Bronchiectasis (ORBIT‐3). ClinicalTrials.gov 2012.
NCT01538667 {published data only}
-
- NCT01538667. Study to characterize lung deposition, pharmacokinetics, safety and tolerability of single inhalations of radiolabeled ciprofloxacin dry powder in healthy subjects and patients with chronic lung diseases [Non‐blinded, single dose, single centre trial to assess the pulmonary deposition as well as pharmacokinetics, safety and tolerability of 99mTc labeled ciprofloxacin when delivered as a single dose from a dry powder inhaler to healthy subjects with and without charcoal block and patients suffering from bronchiectasis and COPD]. clinicaltrials.gov/ct2/show/NCT01538667 (first received 24 February 2012).
NCT01761214 {published data only}
-
- NCT01761214. Bacteriology and inflammation in bronchiectasis [Bacteriology and sputum and systemic inflammation in steady‐state, acute exacerbation and recovery of bronchiectasis]. clinicaltrials.gov/ct2/show/NCT01761214 (first received 4 January 2013).
NCT02035488 {published data only}
-
- NCT02035488. Pharmacokinetic evaluation and tolerability of dry powder tobramycin by a novel device in patients with non cystic fibrosis bronchiectasis. clinicaltrials.gov/ct2/show/NCT02035488 (first received 14 January 2014).
NCT02047773 {published data only}
-
- NCT02047773. Bacterial load guided therapy for severe bronchiectasis exacerbations [Bacterial load guided therapy for severe exacerbations of bronchiectasis requiring intravenous antibiotic therapy‐ BLT Br IV study]. clinicaltrials.gov/ct2/show/NCT02047773 (first received 28 January 2014).
NCT02081963 {published data only}
-
- NCT02081963. Combined administration of nebulized amikacin in patients with acute exacerbation of non‐cystic fibrosis bronchiectasis [A randomized, controlled study of combined administration of nebulized amikacin in patients with acute exacerbation of non‐cystic fibrosis bronchiectasis]. clinicaltrials.gov/ct2/show/NCT02081963 (first received 7 March 2014).
NCT02102152 {published data only}
-
- NCT02102152. Efficacy & tolerability of tobramycin podhaler in bronchiectasis patients with chronic pseudomonas aeruginosa infection. clinicaltrials.gov/ct2/show/NCT02102152 (first received 2 April 2014).
NCT02104245 {published data only}
-
- NCT02104245. Phase 3 Study With Ciprofloxacin Dispersion for Inhalation in Non‐CF Bronchiectasis (ORBIT‐4). ClinicalTrials.gov 2014.
NCT02107274 {published data only}
-
- NCT02107274. Efficacy of azithromycin in treatment of bronchiectasis. clinicaltrials.gov/ct2/show/NCT02107274 (first received 8 April 2014).
NCT02315547 {published data only}
-
- NCT02315547. Sputum microbiota and the association with clinical parameters in steady‐state, acute exacerbation and convalescence of bronchiectasis (BISER‐2). clinicaltrials.gov/ct2/show/NCT02315547 (first received 12 December 2014).
NCT02491723 {published data only}
-
- NCT02491723. Macrolide mediates pulmonary infection of pseudomonas aeruginosa [Macrolide mediates pulmonary infection of pseudomonas aeruginosa via NLRC4 inflammasome signaling pathway]. clinicaltrials.gov/ct2/show/NCT02491723 (first received 8 July 2015).
NCT02509091 {published data only}
-
- NCT02509091. Therapy of bronchoalveolar lavage and local amikacin injection in patients with acute exacerbation of bronchiectasis [Clinical efficacy and safety of therapy of bronchoalveolar lavage and local amikacin injection in patients with acute exacerbation of bronchiectasis:an open‐label randomized parallel controlled study]. clinicaltrials.gov/ct2/show/NCT02509091 (first received 27 July 2015).
NCT02657473 {published data only}
-
- NCT02657473. Inhaled nebulized tobramycin in non‐cystic fibrosis bronchiectasis (BATTLE) [Long‐term inhaled nebulized tobramycin in patients with non‐cystic fibrosis bronchiectasis. A randomized placebo controlled trial. The BATTLE study bronchiectasis and tobramycin solution inhalation therapy]. clinicaltrials.gov/ct2/show/NCT02657473 (first received 15 January 2016).
NCT02661438 {published data only}
-
- NCT02661438. Summative usability study of ciprofloxacin dry powder for inhalation using placebo [Multicenter summative usability study of ciprofloxacin dry powder for inhalation in subjects with non‐cystic fibrosis bronchiectasis (NCFB) or chronic obstructive pulmonary disease (COPD) using matching placebo]. clinicaltrials.gov/ct2/show/NCT02661438 (first received 22 January 2015).
NCT03058718 {published data only}
-
- NCT03058718. Procalcitonin‐guided antibiotic therapy in bronchiectasis [Effectiveness of procalcitonin‐guided antibiotic therapy in acute exacerbations of bronchiectasis: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT03058718 (first received 23 February 2017).
NCT03093974 {published data only}
-
- NCT03093974. Trial in non‐cystic fibrosis bronchiectasis patients with chronic lung infections treated with colistimethate sodium (PROMIS‐I). clinicaltrials.gov/ct2/show/NCT03093974 (first received 28 March 2017).
NCT03460704 {published data only}
-
- Trial in non‐cystic fibrosis bronchiectasis patients with chronic lung infections treated with colistimethate sodium (PROMIS‐2). https://clinicaltrials.gov/show/NCT03460704.
Neumayr 1963 {published data only}
-
- Neumayr A, Stoefler G. Clinical trials of the new antibiotic, rifamycin sv: preliminary report. Chemotherapy 1963;12:437‐8. - PubMed
O'Donnell 2016 {published data only}
-
- O'Donnell AE, Bilton D, Serisier D, Wanner A, Froehlich J, Bruinenberg P, et al. ORBIT‐3 and ORBIT‐4: design of a phase 3 program to investigate safety and efficacy of pulmaquin in non‐cystic fibrosis bronchiectasis (NCFBE) patients chronically colonized with pseudomonas aeruginosa (PA). American Journal of Respiratory and Critical Care Medicine 2016;193:A1775.
Obana 1992 {published data only}
-
- Obana M, Matsuoka Y, Irimajiri S, Yoshioka I. Clinical study on ME1207 in respiratory tract infections. Chemotherapy 1992;40:700‐2.
Odagiri 1992 {published data only}
-
- Odagiri S, Suzuki K, Murohashi K, Takahashi H, Takahashi K. Clinical study of ME 1207 in respiratory tract infections. Chemotherapy 1992;40:703‐7.
Oizumi 1978 {published data only}
-
- Oizumi K, Watanabe A, Konno K, Hayashi I, Kawamorida K, Nagai K, et al. Therapeutic effect of amikacin for infections with gram‐negative bacilli, especially for stubborn respiratory infections (author's transl). [Japanese]. Japanese Journal of Antibiotics 1978;31(1):15‐23. - PubMed
Pezza 1983 {published data only}
-
- Pezza A, Giacomelli P, Montella R, Casino G. Clinical study of a new cephalosporin: ceftriaxone (RO 13‐9904) in resurgent acute and chronic bronchopneumopathies. [Italian]. Archivio Monaldi per la Tisiologia e le Malattie dell'Apparato Respiratorio 1983;38(1):17‐24. - PubMed
Rikitomi 1988 {published data only}
-
- Rikitomi N, Shishido H, Nagatake T, Mbaki U, Matsumoto K. Preclinical and clinical studies on TE‐031 (A‐56268) in treatment of bacterial respiratory tract infections. Chemotherapy 1988;36:715‐28.
Saito 1993 {published data only}
-
- Saito A, Kusano N, Fukuhara H, Soejima R, Nakahama C, Niki Y, et al. A study of the clinical effects of clindamycin on respiratory infections ‐ Focusing on inhibition of beta‐lactamase production. [Japanese]. Chemotherapy 1993;41(11):1232‐45.
Santiveri 1995 {published data only}
-
- Santiveri C, Orriols R, Roig J, Balcells E, Bellver P, Ferrer A, et al. Effectiveness of inhaled antibiotic treatment for pseudomonas aeruginosa in outpatients with colonized bronchiectasis without mucoviscidosis. Archivos de Bronconeumologia 1995;31:42.
Schulz 1972 {published data only}
-
- Schulz G. Comparative studies on the toxicity of tetracyclines and chloramphenicol in the treatment of nonspecific bronchopulmonary infections. [German]. Zeitschrift fur Erkrankungen der Atmungsorgane Mit Folia Bronchologica 1972;136(2):185‐96. - PubMed
Serisier 2011 {published data only}
-
- Serisier DJ, Thompson PJ, Greville H, Kolbe J, Bruinenberg PR. Dual release ciprofloxacin for inhalation (DRCFI) reduces sputum pseudomonas aeruginosa (Pa) density and delays time to infective pulmonary exacerbation in non‐cystic fibrosis (CF) bronchiectasis (BE) [Abstract]. European Respiratory Journal 2011;28:334s.
Serisier 2012 {published data only}
-
- Burr LD, Rogers GB, Chen AC‐H, Hamilton BR, Pool GF, Taylor SL, et al. Macrolide treatment inhibits pseudomonas aeruginosa quorum sensing in non‐cystic fibrosis bronchiectasis: an analysis from the bronchiectasis and low‐dose erythromycin study trial. Annals of the American Thoracic Society 2016;13:1697‐1703. - PubMed
-
- Rogers GB, Bruce KD, Martin ML, Burr LD, Serisier DJ. Corrections to, The effect of long‐term macrolide treatment on respiratory microbiota composition in non‐cystic fibrosis bronchiectasis: an analysis from the randomised, double‐blind, placebo‐controlled BLESS trial. Lancet Respiratory Medicine 2015;3(4):e15. [DOI: 10.1016/S2213-2600(15)00102-2] - DOI - PubMed
-
- Serisier DJ, Bowler SD, McGuckin M, Chen A, Lourie R, Martin ML. The bronchiectasis and low‐dose erythromycin study (BLESS) [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185:A6862.
Shigeno 1984 {published data only}
-
- Shigeno Y, Masaki M, Nakazato H. Laboratory and clinical studies on lenampicillin (KBT‐1585) a new penicillin antibiotic. [Japanese]. Chemotherapy 1984;32:382‐96.
Shimada 1988 {published data only}
-
- Shimada K, Inamatsu T, Fukayama M, Kato A, Adachi K, Sasaki M, et al. Antimicrobial activity and clinical evaluation of NY‐198. Chemotherapy 1988; Vol. 36:537‐44.
Shimokata 1992 {published data only}
-
- Shimokata K, Sakai S, Senda Y, Nishiwaki K, Yamaoto M, Murate T, et al. Evaluation of cefclidin on respiratory tract infections. Chemotherapy 1992;40:748‐52.
Shishido 1995 {published data only}
-
- Shishido H, Furukawa K, Nagai H, Kawakami K, Kono H. Oral levofloxacin 600 mg and 300 mg daily doses in difficult‐to‐treat respiratory infections. Drugs 1995;49:433‐5. - PubMed
Simioli 2017 {published data only}
-
- Simioli F, Sorrentino N, Martino M, Porzio M, Stanziola AA, Molino A, et al. Open label case‐control study to assess Pidotimod efficacy in Non CF Bronchiectasis Disease: a pilot study. European Respiratory Journal 2017;50:PA4063.
Soejima 1988 {published data only}
-
- Soejima R, Niki Y, Hino J, Nakagawa Y, Sumi M, Kishimoto T, et al. Clinical studies on TE‐031 (A‐56268). Chemotherapy 1988;36:673‐8.
Suga 1988 {published data only}
-
- Suga M, Ando M, Tanaka F, Ito K, Sakata T, Sugimoto M, et al. Clinical studies of T‐3262 in respiratory tract infection. [Japanese]. Chemotherapy 1988;36:694‐8.
Sun 2015 {published data only}
-
- Sun JX, Xi MJ, Tu CL, Liu RR, Hu JR, Zhu LP. Efficacy of Juqin mixture aerosol inhalation treatment of bronchiectasis. Chinese Journal of Experimental Traditional Medical Formulae 2015;21:167‐70.
Suyama 1988 {published data only}
-
- Suyama N, Inoue Y, Masaki M, Mashimoto H, Masuyama Y, Dohtsu Y, et al. Laboratory and clinical studies on cefodizime (THR‐221). Chemotherapy 1988;36:529‐44.
Tagaya 2001 {published data only}
-
- Tagaya E, Tamaoki A, Kondo M, Nakada J, Nagai A. Effect and action mechanism of short‐term administration of clarithromycin for airway hypersecretion. [Japanese]. Japanese Journal of Antibiotics 2001;54:33‐35. - PubMed
Tamura 1992 {published data only}
-
- Tamura S, Tokita K, Tsutsumi Z, Ohkawa T, Fujioka H, Kurabori J, et al. Clinical studies on cefclidin. Chemotherapy 1992;40:737‐47.
Tanimoto 1992 {published data only}
-
- Tanimoto H, Obara K, Komatuzaki K, Tanabe O, Okamura T. A clinical study on ME1207 in respiratory infections. Chemotherapy 1992;40:683‐5.
Tanimoto 1993 {published data only}
-
- Tanimoto H, Obara K, Tateishi O, Okamura T. Clinical studies on S‐1108 in respiratory infections. Chemotherapy 1993;41:751‐4.
Twiss 2009 {published data only}
-
- Twiss J, Byrnes C. Nebulised antibiotics reduce symptoms, bacterial density and oral antibiotic usage in children with non cystic fibrosis bronchiectasis [Abstract]. Respirology 2009;14:A76.
Wada 1992 {published data only}
-
- Wada K, Kawashima T, Tukada H, Igarashi K, Arakawa M. Clinical study of ME1207. Chemotherapy 1992;40:708‐10.
Watanabe 1988 {published data only}
-
- Watanabe K, Koyama M, Yokozawa M. Basic and clinical studies on cefotiam hexetil. Chemotherapy 1988;36:311‐22.
Watanabe 1991 {published data only}
-
- Watanabe A. Once daily versus every two week multidose ofloxacin in patients with acute exacerbations of chronic respiratory disease. Infection 1991;19:S384‐7. - PubMed
Watanabe 1995 {published data only}
-
- Watanabe A, Oizumi K, Motomiya M, Nukiwa T. Daily single‐dose regimen and alternate‐two‐week triple‐dose/day regimen of oral ofloxacin for the prophylaxis and control of exacerbations of chronic respiratory tract infections. Tohoku Journal of Experimental Medicine 1995;176(1):25‐33. - PubMed
Weston 1966 {published data only}
-
- Weston M. A clinical trial of fusafungin. British Journal of Diseases of the Chest 1966; Vol. 60, issue 2:104‐6. - PubMed
Wilson 2011 {published data only}
Wong 2004 {published data only}
-
- Wong M, Lam J, Ip S, Lam CL, Tsang KW. Quality of life effect of levofloxacin compared to ceftazidime treatment in infective exacerbation of bronchiectasis [Abstract]. Respirology 2004;9:A135.
Yamada 1993 {published data only}
-
- Yamada H, Katoh O, Aoki Y, Nakata H, Yamaguchi M. Clinical study on S‐1108 in respiratory tract infections. Chemotherapy 1993;41:816‐9.
Yoshida 1989 {published data only}
-
- Yoshida T, Tao M, Tanaka H, Morito T, Nagatake T, Shishido H, et al. Basic and clinical evaluation of cefdinir, a new oral cephem. Chemotherapy 1989;37:579‐90.
Zegaya 1981 {published data only}
-
- Zegaya M, Belkahia Ch, Ladjimi S. Study of doxycycline in pleuropulmonary affections. La Tunisie Medicale 1981;59(5):401‐7. - PubMed
References to ongoing studies
NCT02712983 {published data only}
-
- Dose‐finding Study to Assess the Efficacy, Safety and Tolerability of Tobramycin Inhalation Powder in Patients With Non‐Cystic Fibrosis Bronchiectasis and Pulmonary P. Aeruginosa Infection (iBEST‐1). Ongoing study 2 February 2017.
Additional references
Altenburg 2013
-
- Altenburg J, Graaff CS, Stienstra Y, Sloos JH, Haren EH, Koppers RJ, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non‐cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013;309(12):1251‐9. [PUBMED: 23532241] - PubMed
Barker 2014
-
- Barker AF, O'Donnell AE, Flume P, Thompson PJ, Ruzi JD, Gracia J, et al. Aztreonam for inhalation solution in patients with non‐cystic fibrosis bronchiectasis (AIR‐BX1 and AIR‐BX2): two randomised double‐blind, placebo‐controlled phase 3 trials. Lancet 2014;2(9):738‐49. [PUBMED: 25154045] - PubMed
Bibby 2015
-
- Bibby S, Milne R, Beasley R. Hospital admissions for non‐cystic fibrosis bronchiectasis in New Zealand. New Zealand Medical Journal 2015;128(1421):30‐8. - PubMed
Brodt 2014
-
- Brodt AM, Stovold E, Zhang L. Inhaled antibiotics for stable non‐cystic fibrosis bronchiectasis: a systematic review. European Respiratory Journal 2014;44(2):382‐93. [PUBMED: 24925920] - PubMed
Chalmers 2012
-
- Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short‐ and long‐term antibiotic treatment reduces airway and systemic inflammation in non‐cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2012;186(7):657‐65. [PUBMED: 22744718] - PubMed
Chalmers 2013
-
- Chalmers JD, Hill AT. Mechanisms of immune dysfunction and bacterial persistence in non‐cystic fibrosis bronchiectasis. Molecular Immunology 2013;55(1):27‐34. [PUBMED: 23088941] - PubMed
Chalmers 2014
Chalmers 2015
-
- Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. European Respiratory Journal 2015;45(5):1446‐62. [PUBMED: 25792635] - PubMed
Chang 2002
-
- Chang AB, Grimwood K, Mulholland EK, Torzillo PJ. Bronchiectasis in indigenous children in remote Australian communities. Medical Journal of Australia 2002;177(4):200‐4. [PUBMED: 12175325] - PubMed
Chang 2010
-
- Chang AB, Bell SC, Byrnes CA, Grimwood K, Holmes PW, King PT, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand. Medical Journal of Australia 2010;193(6):356‐65. [PUBMED: 20854242] - PubMed
Cole 1986
-
- Cole PJ. Inflammation: a two‐edged sword ‐ the model of bronchiectasis. European Journal of Respiratory Diseases. Supplement 1986;147:6‐15. [PUBMED: 3533593] - PubMed
Cole 1997
-
- Cole P. The damaging role of bacteria in chronic lung infection. Journal of Antimicrobial Chemotherapy 1997;40 Suppl A:5‐10. [PUBMED: 9484867] - PubMed
de la Rosa 2016
-
- Rosa D, Martínez‐García MA, Olveira C, Girón R, Máiz L, Prados C. Annual direct medical costs of bronchiectasis treatment: Impact of severity, exacerbations, chronic bronchial colonization and chronic obstructive pulmonary disease coexistence. Chronic Respiratory Disease 2016;13(4):361‐71. [PUBMED: 27072020] - PMC - PubMed
Edwards 2000
-
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000;356(9237):1255‐9. [PUBMED: 11072960] - PubMed
Elborn 2016
-
- Elborn JS, Vataire A‐L, Fukushima A, Aballea S, Khemiri A, Moore C, et al. Comparison of inhaled antibiotics for the treatment of chronic pseudomonas aeruginosa lung Infection in patients with cystic fibrosis: systematic literature review and network meta‐analysis. Clinical Therapeutics 2016;8(8):1‐23. - PubMed
European Lung White Book 2013
-
- Gibson GJ, Loddenkemper R, Sibille Y, Lundbäck B, editor(s). European Lung White Book: Respiratory Health and Disease in Europe. European Lung White Book: Respiratory Health and Disease in Europe. European Respiratory Society, 2013. [www.erswhitebook.org] - PubMed
Evans 1996
-
- Evans SA, Turner SM, Bosch BJ, Hardy CC, Woodhead MA. Lung function in bronchiectasis: the influence of Pseudomonas aeruginosa. European Respiratory Journal 1996;9(8):1601‐4. [PUBMED: 8866579] - PubMed
Finch 2015
-
- Finch S, McDonnell MJ, Abo‐Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Annals of the American Thoracic Society 2015;12(11):1602‐11. [PUBMED: 26356317] - PubMed
Foweraker 2011
-
- Foweraker J, Wat D. Microbiology of non‐CF bronchiectasis [Bronchiectasis]. In: Floto RA, Haworth CS editor(s). European Respiratory Society Monographs. Vol. 52, European Respiratory Society, 2011:68‐96.
Goeminne 2016
-
- Goeminne PC, Soyza A. Bronchiectasis: how to be an orphan with many parents?. European Respiratory Journal 2016;47(1):10‐3. [PUBMED: 26721955] - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 30 January 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Habesoglu 2011
Hansen 2015
Haworth 2014
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 2017
-
- Hill AT, Haworth CS, Aliberti S, Barker A, Blasi F, Boersma W, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. European Respiratory Journal 2017;49(6):pii: 1700051. - PubMed
Hnin 2015
Hui 2013
Joish 2013
-
- Joish VN, Spilsbury‐Cantalupo M, Operschall E, Luong B, Boklage S. Economic burden of non‐cystic fibrosis bronchiectasis in the first year after diagnosis from a US health plan perspective. Applied Health Economics and Health Policy 2013;11(3):299‐304. [PUBMED: 23580074] - PubMed
Kapur 2012
-
- Kapur N, Masters IB, Newcombe P, Chang AB. The burden of disease in pediatric non‐cystic fibrosis bronchiectasis. Chest 2012;141(4):1018‐24. [PUBMED: 21885727] - PubMed
Littlewood 2012
Martínez‐García 2007
-
- Martínez‐García MA, Soler‐Cataluña JJ, Perpìñá‐Tordera M, Román‐Sánchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non‐cystic fibrosis bronchiectasis. Chest 2007;132(5):1565‐72. [PUBMED: 17998359] - PubMed
Martínez‐García 2018
McCullough 2014
Melnyk 2015
Moher 2009
Murray 2011
-
- Murray MP, Govan JRW, Doherty CJ, Simpson AJ, Wilkinson TS, Chalmers JD, et al. A randomized controlled trial of nebulized gentamicinin non–cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2011;183:491–9. - PubMed
Pasteur 2010
-
- Pasteur MC, Bilton D, Hill AT, British Thoracic Society Bronchiectasis (non‐CF) Guideline Group. British Thoracic Society Guidelines for non‐CF bronchiectasis. Thorax 2010;65(Suppl1):i1–58. - PubMed
Polverino 2017
Quint 2016
Redondo 2016
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ringshausen 2015
-
- Ringshausen FC, Roux A, Diel R, Hohmann D, Welte T, Rademacher J. Bronchiectasis in Germany: a population‐based estimation of disease prevalence. European Respiratory Journal 2015;46(6):1805‐7. [PUBMED: 26293498] - PubMed
Roberts 2010
-
- Roberts HJ, Hubbard R. Trends in bronchiectasis mortality in England and Wales. Respiratory Medicine 2010;104:981‐5. - PubMed
Rogers 2014
-
- Rogers GB, Zain NM, Bruce KD, Burr LD, Chen AC, Rivett DW, et al. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Annals of the American Thoracic Society 2014;11(4):496‐503. [PUBMED: 24592925] - PubMed
Seitz 2010
Serisier 2013
-
- Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, Brain B, et al. Effect of long‐term, low‐dose erythromycin on pulmonary exacerbations among patients with non‐cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013;309(12):1260‐7. [PUBMED: 23532242] - PubMed
Twiss 2005
Weycker 2005
-
- Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clinical Pulmonary Medicine 2005;12(4):205‐9.
Wilson 1997
-
- Wilson CB, Jones PW, O'Leary CJ, Hansell DM, Cole PJ, Wilson R. Effect of sputum bacteriology on the quality of life of patients with bronchiectasis. European Respiratory Journal 1997;10(8):1754‐60. [PUBMED: 9272915] - PubMed
Wilson 2013
Wong 2012
-
- Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin for prevention of exacerbations in non‐cystic fibrosis bronchiectasis (EMBRACE): a randomised, double‐blind, placebo‐controlled trial. Lancet 2012;380(9842):660‐7. [PUBMED: 22901887] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous