Post-Exposure Protection in Mice against Sudan Virus by a Two Antibody Cocktail
- PMID: 29861435
- PMCID: PMC6024315
- DOI: 10.3390/v10060286
Post-Exposure Protection in Mice against Sudan Virus by a Two Antibody Cocktail
Abstract
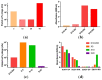
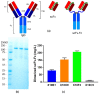
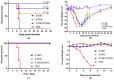
Sudan virus (SUDV) and Ebola viruses (EBOV) are both members of the Ebolavirus genus and have been sources of epidemics and outbreaks for several decades. We present here the generation and characterization of cross-reactive antibodies to both SUDV and EBOV, which were produced in a cell-free system and protective against SUDV in mice. A non-human primate, cynomolgus macaque, was immunized with viral-replicon particles expressing the glycoprotein of SUDV-Boniface (8A). Two separate antibody fragment phage display libraries were constructed after four immunogen injections. Both libraries were screened first against the SUDV and a second library was cross-selected against EBOV-Kikwit. Sequencing of 288 selected clones from the two distinct libraries identified 58 clones with distinct VH and VL sequences. Many of these clones were cross-reactive to EBOV and SUDV and able to neutralize SUDV. Three of these recombinant antibodies (X10B1, X10F3, and X10H2) were produced in the scFv-Fc format utilizing a cell-free production system. Mice that were challenged with SUDV-Boniface receiving 100µg of the X10B1/X10H2 scFv-Fc combination 6 and 48-h post-exposure demonstrated partial protection individually and complete protection as a combination. The data herein suggests these antibodies may be promising candidates for further therapeutic development.
Keywords: Ebola; Sudan virus; antibody; biodefense; cell-free production; phage display; protection.
Conflict of interest statement
The authors declare no conflict of interest. J.W.F. now serves in a science and technology management role at the Defense Threat Reduction Agency but this assignment was after the design and completion of the work in this manuscript. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Kuhn R. Togaviridae: The Viruses and Their Replication. In: Knipe D.M., editor. Fields Virology. 5th ed. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

