Kinetics and mechanism of the oxidation of a cobaloxime by sodium hypochlorite in aqueous solution: Is it an outer-sphere mechanism?
- PMID: 29861504
- PMCID: PMC5976256
- DOI: 10.1016/j.ica.2016.07.013
Kinetics and mechanism of the oxidation of a cobaloxime by sodium hypochlorite in aqueous solution: Is it an outer-sphere mechanism?
Abstract
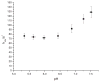
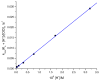
The kinetics and mechanism of the oxidation of [Co(dmgBF2)2(OH2)2] (where dmgBF2 = difluoroboryldimethylglyoximato) by sodium hypochlorite (NaOCl) were investigated by stopped-flow spectrophotometry at 450 nm over the temperature range of 10 °C ≤ θ ≤ 25 °C, pH range of 5.0 ≤ pH ≤ 7.8, and at an ionic strength of 0.60 M (NaCl). The pKa1 value for [Co(dmgBF2)2(H2O)2] was calculated as 5.27 ± 0.14 at I = 0.60 (NaCl). The redox process was dependent on pH and oxidant concentration in a complex manner, that is, kobs = ((k2[H+] + k1Ka)/([H+] + Ka))[OCl-]T, where at 25.3 °C, k1 was calculated as 3.54 × 104 M-1 s-1, and k2 as 2.51 × 104 M-1 cm-1. At a constant pH value, while varying the concentration of sodium hypochlorite two rate constants were calculated, viz., k'1 = 7.56 s-1 (which corresponded to a reaction pathway independent of the NaOCl concentration) and k'2 = 2.26 × 104 M-1 s-1, which was dependent on the concentration of NaOCl. From the variation in pH, [Formula: see text], and [Formula: see text] were calculated as 58 ± 16 kJ mol-1, 46 ± 1 kJ mol-1, 34 ± 55 J mol-1 K-1, and -6 ± 4 Jmol-1 K-1, respectively. The self-exchange rate constant, k11, for sodium hypochlorite (as ClO-) was calculated to be 1.2 × 103 M-1 s-1, where an outer-sphere electron transfer mechanism was assumed. A green product, [Co(dmgBF2)2(OH2)(OH)]·1.75NaOCl, which can react with DMSO, was isolated from the reaction at pH 8.04 with a yield of 13%.
Keywords: 59Co NMR spectroscopy; Cobaloximes; Kinetics; Self-exchange rate constants; Sodium hypochlorite.
Figures












References
-
- Armaroli N, Balzani V. Angew Chem Int Ed. 2007;46:52–66. - PubMed
-
- Eisenberg R, Nocera DG. Inorg Chem. 2005;44:6799–6801. - PubMed
-
- Hoffert MI, Caldeira K, Jain AK, Haites EF, Harvey LDD, Potter SD, Schlesinger ME, Schneider SH, Watts RG, Wigley TML, Wuebbles DJ. Nature. 1998;395:881–884.
-
- Gray HB. Nat Chem. 2009;1:7–7. - PubMed
-
- Lubitz W, Tumas W. Chem Rev. 2007;107:3900–3903. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
