Properties and applications of nanoparticle/microparticle conveyors with adjuvant characteristics suitable for oral vaccination
- PMID: 29861631
- PMCID: PMC5968786
- DOI: 10.2147/IJN.S154743
Properties and applications of nanoparticle/microparticle conveyors with adjuvant characteristics suitable for oral vaccination
Abstract
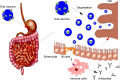
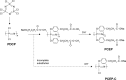
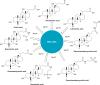
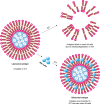
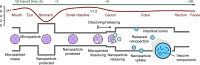
Vaccination is one of the most effective approaches in the prevention and control of disease worldwide. Oral vaccination could have wide applications if effective protection cannot be achieved through traditional (eg, parenteral) routes of vaccination. However, oral administration is hampered by the difficulties in transferring vaccines in vivo. This has led to the development of materials such as carriers with potential adjuvant effects. Considering the requirements for selecting adjuvants for oral vaccines as well as the advantages of nanoparticle/microparticle materials as immune effectors and antigen conveyors, synthetic materials could improve the efficiency of oral vaccination. In this review, nanoparticles and microparticles with adjuvant characteristics are described with regard to their potential importance for oral immunization, and some promising and successful modification strategies are summarized.
Keywords: NP/MP conveyors; antibody; immune response; intestine; oral vaccine.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

