Asymmetric Michael Addition Mediated by Chiral Ionic Liquids
- PMID: 29861702
- PMCID: PMC5925874
- DOI: 10.2174/1570193X15666171211165344
Asymmetric Michael Addition Mediated by Chiral Ionic Liquids
Abstract
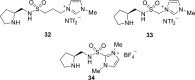
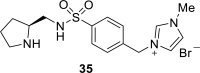
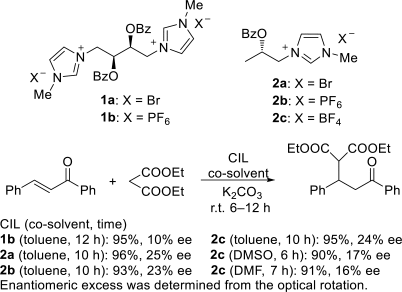
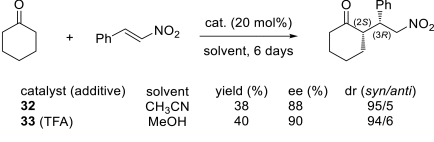
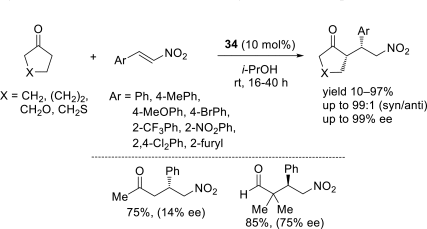
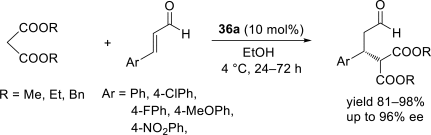
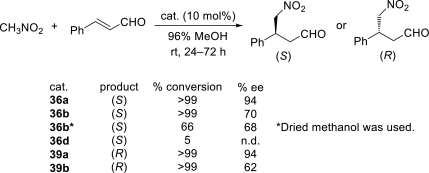
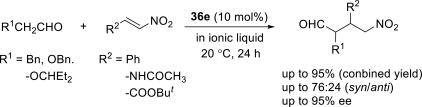
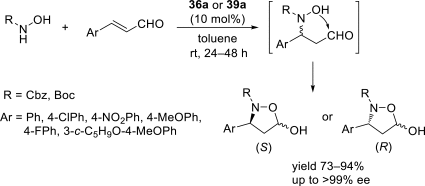
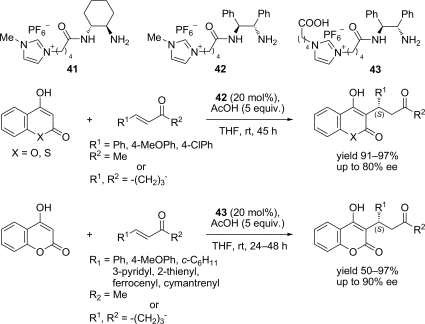
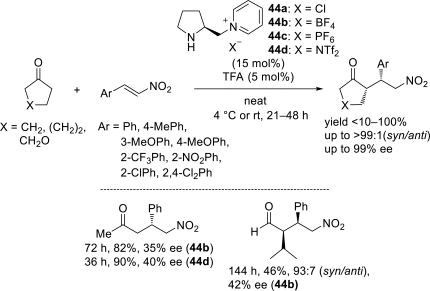
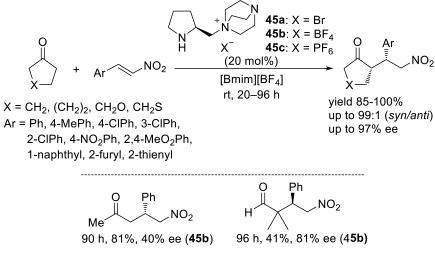
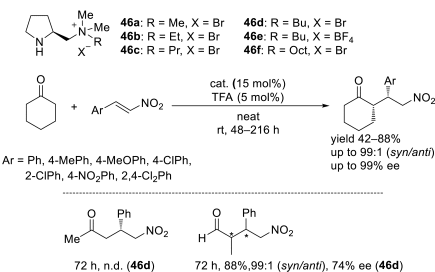
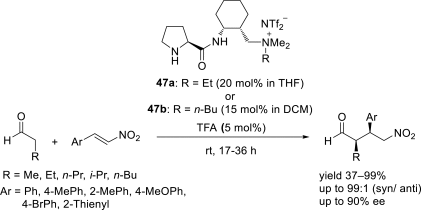
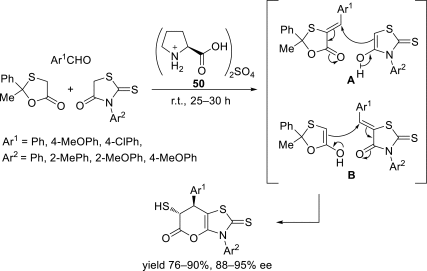
Chiral ionic liquids with a focus on their applications in asymmetric Michael additions and related reactions were reviewed. The examples were classified on the basis of the mode of asymmetric induction (e.g., external induction/non-covalent interaction or internal induction/covalent bond formation), the roles in reactions (as a solvent or catalyst), and their structural features (e.g., imidazolium-based chiral cations, other chiral oniums; proline derivatives). Most of the reactions with high chiral induction are Michael addition of ketones or aldehydes to chalcones or nitrostyrenes where proline-derived chiral ionic liquids catalyze the reaction through enamine/ iminium formation. Many reports demonstrate the recyclability of ionic liquid-tagged pyrrolidines.
Keywords: Chiral ionic liquid; Michael addition; asymmetric reaction; catalysts; chiral solvent; imidazole ionic liquid.
Figures
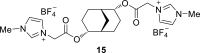
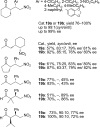
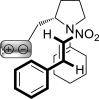

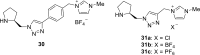
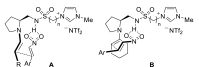


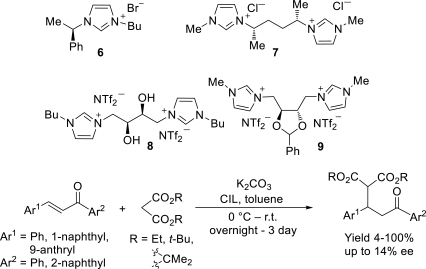
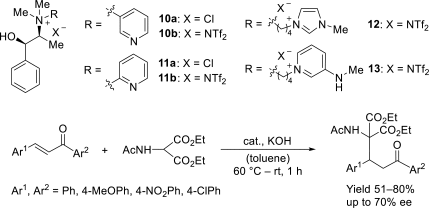
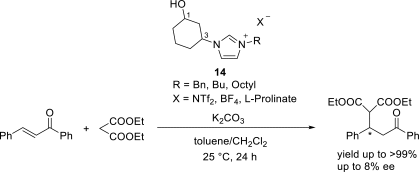



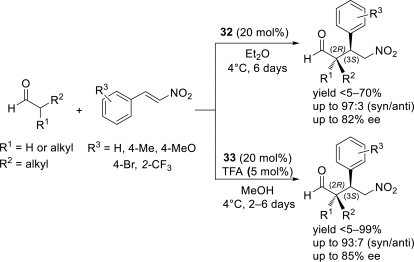



References
-
- Earle M.J., McCormac P.B., Seddon K.R. Diels-Alder reactions in ionic liquids. A safe recyclable alternative to lithium perchlorate-diethyl ether mixtures. Green Chem. 1999;1(1):23–25.
-
- Baudequin C., Bregeon D., Levillain J., Guillen F., Plaquevent J-C., Gaumont A-C. Chiral ionic liquids, a renewal for the chemistry of chiral solvents? Design, synthesis and applications for chiral recognition and asymmetric synthesis. Tetrahedron Asymmetry. 2005;16(24):3921–3945.
-
- Ding J., Armstrong D.W. Chiral ionic liquids: Synthesis and applications. Chirality. 2005;17(5):281–292. - PubMed
-
- Headley A.D., Ni B. Chiral imidazolium ionic liquids: Their synthesis and influence on the outcome of organic reactions. Aldrichim Acta. 2007;40(4):107–117.
-
- Chen X., Li X.I., Hu A., Wang F. Advances in chiral ionic liquids derived from natural amino acids. Tetrahedron Asymmetry. 2008;19(1):1–14.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
