A Simple Procedure for the Synthesis of β-Hydroxyallenamides via Homoallenylation of Aldehydes
- PMID: 29861707
- PMCID: PMC5977997
- DOI: 10.1002/adsc.201800119
A Simple Procedure for the Synthesis of β-Hydroxyallenamides via Homoallenylation of Aldehydes
Abstract
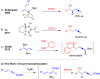
A simple one-pot synthesis of β-hydroxyallenamides is reported. This procedure entails chemo- and regioselective hydroboration of 3-en-1-ynyl-sulfonylamides with Cy2BH followed by homoallenylation of aldehydes to yield β-hydroxyallenamides (up to 94% yield and >20:1 dr). Controlled synthesis of up to three continuous stereochemical elements was realized. Density functional theory (DFT) calculations suggest a concerted Zimmerman-Traxler chair-like transition state. Initial results suggest that enantio- and diastereoselective synthesis of β-hydroxyallenamides with optically active hydroboration reagents is viable.
Keywords: Allenamide; Enynamide; Homoallenylation; Hydroboration; Zimmerman-Traxler Transition State.
Figures




References
-
- Helmechen RHG, Mulzer J, Schaumann E. Stereoselective Synthesis Methods of Organic Chemistry (Houben-Weyl) E21. Thieme; Stuggart: 1996.
- Yamamoto Y, Asao N. Chem Rev. 1993;93:2207.
- Denmark SE, Fu J. J Am Chem Soc. 2000;122:12021.
- Denmark SE, Fu J. Chem Rev. 2003;103:2763. - PubMed
- Lachance H, Hall DG. Allylboration of Carbonyl Compounds in Organic Reactions, Vol. Vol. 73. John Wiley & Sons, Inc; 2008. p. 1.
-
- Chen M, Handa M, Roush WR. J Am Chem Soc. 2009;131:14602. - PMC - PubMed
- Guzman-Martinez A, Hoveyda AH. J Am Chem Soc. 2010;132:10634. - PMC - PubMed
- Chen M, Ess DlH, Roush WR. J Am Chem Soc. 2010;132:7881. - PMC - PubMed
- Fernández E, Pietruszka J, Frey W. J Org Chem. 2010;75:5580. - PubMed
- Chen JLY, Scott HK, Hesse MJ, Willis CL, Aggarwal VK. J Am Chem Soc. 2013;135:5316. - PubMed
- Incerti-Pradillos CA, Kabeshov MA, Malkov AV. Angew Chem Int Ed. 2013;52:5338. - PubMed
- Angew Chem. 2013;125:4510.
- Horino Y, Aimono A, Abe H. Org Lett. 2015;17:2824. - PubMed
-
- Soundararajan R, Li G, Brown HC. J Org Chem. 1996;61:100.
-
-
For reviews on boron-substituted 1,3-dienes, see:
- Hilt G, Bolze P. Synthesis. 2005;2091
- Hansen EC, Lee D. Acc Chem Res. 2006;39:509. - PubMed
- Welker ME. Tetrahedron. 2008;64:11529.
- Cornil J, Guerinot A, Cossy J. Org Biomol Chem. 2015;13:4129. - PubMed
- Pyziak J, Walkowiak J, Marciniec B. Chem Eur J. 2017;23:3502. - PubMed
-
-
- Renaud J, Graf CD, Oberer L. Angew Chem Int Ed. 2000;39:3101. - PubMed
- Angew Chem. 2000;112:323.
- Micalizio GC, Schreiber SL. Angew Chem Int Ed. 2002;41:3272. - PubMed
- Angew Chem. 2002;114:3406.
- Shimizu M, Nakamaki C, Shimono K, Schelper M, Kurahashi T, Hiyama T. J Am Chem Soc. 2005;127:12506. - PubMed
- Kim M, Lee D. Org Lett. 2005;7:1865. - PubMed
- Botvinik A, Quntar AAA, Rubinstein A, Srebnik M. J Organomet Chem. 2009;694:3349.
- Xu S, Lee CT, Rao H, Negishi EI. Adv Synth Catal. 2011;353:2981. - PMC - PubMed
- Wang L, Welker ME. J Org Chem. 2012;77:8280. - PubMed
- Semba K, Fujihara T, Terao J, Tsuji Y. Angew Chem Int Ed. 2013;52:12400. - PubMed
- Angew Chem. 2013;125:12626.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
