Chronic Inflammation May Enhance Leiomyoma Development by the Involvement of Progenitor Cells
- PMID: 29861738
- PMCID: PMC5971255
- DOI: 10.1155/2018/1716246
Chronic Inflammation May Enhance Leiomyoma Development by the Involvement of Progenitor Cells
Abstract
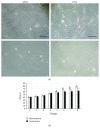
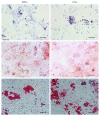
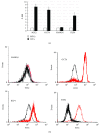
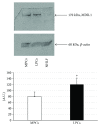
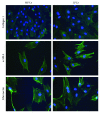
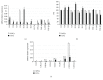
Although the etiology of leiomyoma is unclear, a progenitor/undifferentiated cell population has been described whose dysregulation may be involved in the onset of uterine conditions. Moreover, inflammation is involved in the development of several tumors. The aim of this work was to understand if progenitor cells sustain a chronic inflammatory microenvironment that enhances leiomyoma development. Cells from 12 human leiomyoma and 12 normal myometrium samples of the same patients were in vitro isolated and exhaustively characterized (morphology, proliferation, cytofluorometry, differentiation, RT-PCR, immunofluorescence, immunohistochemistry, and Western blotting assays). Selected cytokines (ELISA) and inflammation-related genes (RT-PCR) were analyzed to identify healthy myometrium progenitor cells (MPCs) and leiomyoma progenitor cells (LPCs). Results show that (i) MPCs and LPCs share stemness features, such as immunophenotype and multidifferentiation assay, (ii) LPCs have a significantly shorter doubling time and a significantly higher expression of stemness genes (p < 0.05), and (iii) LPCs secreted significantly higher levels (p < 0.05) of cytokines related to chronic inflammation and significantly lower amounts (p < 0.05) of cytokines related to acute inflammation. Despite the limited sample size, comparisons between leiomyoma and normal myometrium tissue from each patient allowed normalization of patient-specific differences. The evidenced cytokine expression pattern related to chronic inflammation in LPCs may play a role in the increased risk of adverse obstetric outcomes (infertility, spontaneous miscarriage, and preterm birth) in women affected by leiomyomas. These women should be recognized as "high risk" and subjected to specialized management both before and during pregnancy.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

