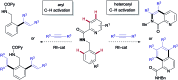
RhI/RhIII catalyst-controlled divergent aryl/heteroaryl C-H bond functionalization of picolinamides with alkynes
- PMID: 29861907
- PMCID: PMC5950197
- DOI: 10.1039/c5sc01885d
RhI/RhIII catalyst-controlled divergent aryl/heteroaryl C-H bond functionalization of picolinamides with alkynes
Abstract
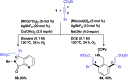
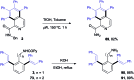
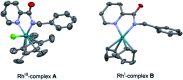
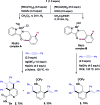
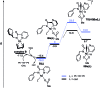
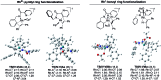
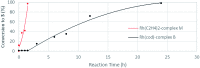
The ability to establish switchable site-selectivity through catalyst control in the direct functionalization of molecules that contain distinct C-H bonds remains a demanding challenge that would enable the construction of diverse scaffolds from the same starting materials. Herein we describe the realization of this goal, namely a divergent heteroaryl/aryl C-H functionalization of aromatic picolinamide derivatives, targeting two distinct C-H sites, either at the pyridine ring or at the arene unit, to afford isoquinoline or ortho-olefinated benzylamine (or phenethylamine) derivatives. This complementary reactivity has been achieved on the basis of a RhIII/RhI switch in the catalyst, resulting in different mechanistic outcomes. Notably, a series of experimental and DFT mechanistic studies revealed important insights about the mechanism of the reaction and reasons behind the divergent regiochemical outcome.
Figures


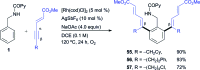






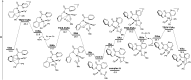
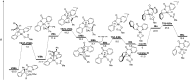

References
-
-
Recent general reviews on C–H functionalization: . See also:
- Wencel-Delord J., Drçge T., Liu F., Glorius F. Chem. Soc. Rev. 2011;40:4740. - PubMed
- Newhouse T., Baran P. S. Angew. Chem., Int. Ed. 2011;50:3362. - PMC - PubMed
- Ackermann L. Chem. Rev. 2011;111:1315. - PubMed
- McMurray L., O'Hara F., Gaunt M. J. Chem. Soc. Rev. 2011;40:1885. - PubMed
- Yeung C. S., Dong V. M. Chem. Rev. 2011;111:1215. - PubMed
- White M. C. Science. 2012;335:807. - PubMed
- Engle K. M., Mei T.-S., Wasa M., Yu J.-Q. Acc. Chem. Res. 2012;45:788. - PMC - PubMed
- Yamaguchi J., Yamaguchi A. D., Itami K. Angew. Chem., Int. Ed. 2012;51:8960. - PubMed
- Neufeldt S. R., Sanford M. S. Acc. Chem. Res. 2012;45:936. - PMC - PubMed
- Kuhl N., Hopkinson M. N., Wencel-Delord J., Glorius F. Angew. Chem., Int. Ed. 2012;51:10236. - PubMed
- Chen D. Y.-K., Youn S. W. Chem.–Eur. J. 2012;18:9452. - PubMed
- Wencel-Delord J., Glorius F. Nat. Chem. 2013;5:369. - PubMed
- Ackermann L. Acc. Chem. Res. 2014;47:281. - PubMed
- Zhang X.-S., Chen K., Shi Z.-J. Chem. Sci. 2014;5:2146.
- Yu D.-G., de Azambuja F., Glorius F. Angew. Chem., Int. Ed. 2014;53:7710. - PubMed
-
-
-
For elegant examples: . For a review on catalytic selective synthesis:
- Campeau L.-C., Chipper D. J., Fagnou K. J. Am. Chem. Soc. 2008;130:3266. - PubMed
- Schipper D. J., Campeau L.-C., Fagnou K. Tetrahedron. 2009;65:3155.
- Lapointe D., Markiewicz T., Whipp C. J., Toderian A., Fagnou K. J. Org. Chem. 2011;76:749. - PubMed
- Dooley J. D., Chidipudi S. R., Lam H. W. J. Am. Chem. Soc. 2013;135:10829. - PubMed
- Zhang X., Si W., Bao M., Asao N., Yamamoto Y., Jin T. Org. Lett. 2014;16:4830. - PubMed
- Dooley J. D., Chidipudi S. R., Lam H. W. J. Am. Chem. Soc. 2013;135:10829. - PubMed
- Cross W. B., Razak S., Singh K., Warner A. J. Chem.–Eur. J. 2014;20:13203. - PubMed
- Du W., Gu Q., Li Z., Yang D. J. Am. Chem. Soc. 2015;137:1130. - PubMed
- Bedford R. B., Durrant S. J., Montgomery M. Angew. Chem., Int. Ed. 2015;54 doi: 10.1002/anie.201502150. - DOI - PMC - PubMed
- Kang D., Hong S. Org. Lett. 2015;17:1938. - PubMed
- Mahatthananchai J., Dumas A. M., Bode J. W. Angew. Chem., Int. Ed. 2012;51:10954. - PubMed
-
-
-
For reviews on Rh-catalyzed C–H functionalization:
- Lewis J. C., Bergman R. G., Ellman J. A. Acc. Chem. Res. 2008;41:1013. - PMC - PubMed
- Bouffard J., Itami K. Top. Curr. Chem. 2010;292:231. - PubMed
- Colby D. A., Bergman R. G., Ellman J. A. Chem. Rev. 2010;110:624. - PMC - PubMed
- Bouffard J., Itami K. Top. Curr. Chem. 2010;292:231. - PubMed
- Satoh T., Miura M. Chem.–Eur. J. 2010;16:11212. - PubMed
- Colby D. A., Tsai A. S., Bergman R. G., Ellman J. A. Acc. Chem. Res. 2012;45:814. - PMC - PubMed
- Song G., Wang F., Li X. Chem. Soc. Rev. 2012;41:3651. - PubMed
- Patureau F. W., Wencel-Delord J., Glorius F. Aldrichimica Acta. 2012;45:31.
- Kuhl N., Schröder N., Glorius F. Adv. Synth. Catal. 2014;356:1443.
- Ghorai D., Choudhury J. Acc. Chem. Res. 2015;48:1007.
- Ye B., Cramer N. Acc. Chem. Res. 2015;48:1308. - PubMed
-
-
-
For selected examples of RhIII-catalyzed C–H annulative coupling reactions of (hetero)arenes involving one equivalent of an alkyne:
- Ueura K., Satoh T., Miura M. Org. Lett. 2007;9:1407. - PubMed
- Li L., Brennessel W. W., Jones W. D. J. Am. Chem. Soc. 2008;130:12414. - PubMed
- Stuart D. R., Bertrand-Laperle M., Burgess K. M. N., Fagnou K. J. Am. Chem. Soc. 2008;130:16474. - PubMed
- Guimond N., Fagnou K. J. Am. Chem. Soc. 2009;131:12050. - PubMed
- Fukutani T., Umeda N., Hirano K., Satoh T., Miura M. Chem. Commun. 2009:5141. - PubMed
- Stuart D. R., Alsabeh P., Kuhn M., Fagnou K. J. Am. Chem. Soc. 2010;132:18326. - PubMed
- Rakshit S., Patureau F. W., Glorius F. J. Am. Chem. Soc. 2010;132:9585. - PubMed
- Guimond N., Gouliaras C., Fagnou K. J. Am. Chem. Soc. 2010;132:6908. - PubMed
- Hyster T. K., Rovis T. J. Am. Chem. Soc. 2010;132:10565. - PMC - PubMed
- Morimoto K., Hirano K., Satoh T., Miura M. Org. Lett. 2010;12:2068. - PubMed
- Chen J., Song G., Pan C.-L., Li X. Org. Lett. 2010;12:5426. - PubMed
- Too P. C., Wang Y.-F., Chiba S. Org. Lett. 2010;12:5688. - PubMed
- Muralirajan K., Parthasarathy K., Cheng C.-H. Angew. Chem., Int. Ed. 2011;50:4169. - PubMed
- Patureau F. W., Besset T., Kuhl N., Glorius F. J. Am. Chem. Soc. 2011;133:2154. - PubMed
- Guimond N., Gorelsky S. I., Fagnou K. J. Am. Chem. Soc. 2011;133:6449. - PubMed
- Wang Y.-F., Toh K. K., Lee J.-Y., Chiba S. Angew. Chem., Int. Ed. 2011;50:5927. - PubMed
- Huestis M. P., Chan L., Stuart D. R., Fagnou K. Angew. Chem., Int. Ed. 2011;50:1338. - PubMed
- Zhang X., Chen D., Zhao M., Zhao J., Jia A., Li X. Adv. Synth. Catal. 2011;353:719.
- Wang H., Grohmann C., Nimphius C., Glorius F. J. Am. Chem. Soc. 2012;134:19592. - PubMed
- Jayakumar J., Parthasarathy K., Cheng C.-H. Angew. Chem., Int. Ed. 2012;51:197. - PubMed
- Pham M. V., Ye B., Cramer N. Angew. Chem., Int. Ed. 2012;51:10610. - PubMed
- Xu X., Liu Y., Park C.-M. Angew. Chem., Int. Ed. 2012;51:9372. - PubMed
- Li B.-J., Wang H.-Y., Zhu Q.-L., Shi Z.-J. Angew. Chem., Int. Ed. 2012;51:3948. - PubMed
- Tan X., Liu B., Li X., Li B., Xu S., Song H., Wang B. J. Am. Chem. Soc. 2012;134:16163. - PubMed
- Dong W., Wang L., Parthasarathy K., Pan F., Bolm C. Angew. Chem., Int. Ed. 2013;52:11573. - PubMed
- Liu B., Song C., Sun C., Zhou S., Zhu J. J. Am. Chem. Soc. 2013;135:16625. - PubMed
- Zhao D., Shi Z., Glorius F. Angew. Chem., Int. Ed. 2013;52:12426. - PubMed
- Wang C., Sun H., Fang Y., Huang Y. Angew. Chem., Int. Ed. 2013;52:5795. - PubMed
- Wang C., Huang Y. Org. Lett. 2013;15:5294. - PubMed
- Kim D.-S., Park J.-W., Jun C.-H. Adv. Synth. Catal. 2013;355:2667.
- Zheng L., Hua R. Chem.–Eur. J. 2014;20:2352. - PubMed
- Li X. G., Liu K., Zou G., Liu P. N. Adv. Synth. Catal. 2014;356:1496.
- Muralirajana K., Cheng C.-H. Adv. Synth. Catal. 2014;356:1571.
- Hoshino Y., Shibata Y., Tanaka K. Adv. Synth. Catal. 2014;356:1577.
- Fukui M., Hoshino Y., Satoh T., Miura M., Tanaka K. Adv. Synth. Catal. 2014;356:1638.
- Xing L., Fan Z., Hou C., Yong G., Zhang A. Adv. Synth. Catal. 2014;356:972.
- Jayakumar J., Parthasarathy K., Chen Y.-H., Lee T.-H., Chuang S.-C., Cheng C.-H. Angew. Chem., Int. Ed. 2014;53:9889. - PubMed
- Nobushige K., Hirano K., Satoh T., Miura M. Org. Lett. 2014;16:1188. - PubMed
- Iitsuka T., Hirano K., Satoh T., Miura M. Chem.–Eur. J. 2014;20:385. - PubMed
- Yu D.-G., de Azambuja F., Gensch T., Daniliuc C. G., Glorius F. Angew. Chem., Int. Ed. 2014;53:9650. - PubMed
- Li D. Y., Chen H. J., Liu P. N. Org. Lett. 2014;16:6176. - PubMed
- Seoane A., Casanova N., Quiñones N., Mascareñas J. L., Gulías M. J. Am. Chem. Soc. 2014;136:834. - PubMed
- Jia J., Shi J., Zhou J., Liu X., Song Y., Xu H. E., Yi W. Chem. Commun. 2015;51:2925. - PubMed
- Fan Z., Song S., Li W., Geng K., Xu Y., Miao Z.-H., Zhang A. Org. Lett. 2015;17:310. - PubMed
- Yang X.-F., Hu X.-H., Loh T.-P. Org. Lett. 2015;17:1481. - PubMed
- Ghorai D., Choudhury J. ACS Catal. 2015;5:2692.
- Duttwyler S., Lu C., Rheingold A. L., Bergman R. G., Ellman J. A. J. Am. Chem. Soc. 2012;134:4064. - PMC - PubMed
- Azpíroz R., Di Giuseppe A., Castarlenas R., Pérez-Torrente J. J., Oro L. A. Chem.–Eur. J. 2013;19:3812. - PubMed
- Zhang Q.-R., Huang J.-R., Zhang W., Dong L. Org. Lett. 2014;16:1684. - PubMed
- Luo C.-Z., Jayakumar J., Gandeepan P., Wu Y.-C., Cheng C.-H. Org. Lett. 2015;17:924. - PubMed
- Zhao S., Yu R., Chen W., Liu M., Wu H. Org. Lett. 2015;17:2828. - PubMed
- Yang Y., Zhou M.-B. o., Ouyang X.-H., Pi R., Song R.-J., Li J.-H. Angew. Chem., Int. Ed. 2015;54:6595. - PubMed
- Zheng J., Wang S.-B., Zheng C., You S.-L. J. Am. Chem. Soc. 2015;137:4880. - PubMed
- Dateer R. B., Chang S. J. Am. Chem. Soc. 2015;137:4908. - PubMed
- Qiu L., Huang D., Xu G., Dai Z., Sun J. Org. Lett. 2015;17:1810. - PubMed
- Zhao Y., Han F., Yang L., Xia C. Org. Lett. 2015;17:1477. - PubMed
- Qi Z., Yu S., Li X. J. Org. Chem. 2015;80:3471. - PubMed
- Chen S., Bergman R. G., Ellman J. A. Org. Lett. 2015;17:2567. - PMC - PubMed
- Litsuka T., Hirano K., Satoh T., Miura M. J. Org. Chem. 2015;80:2804. - PubMed
- Jia J., Shi J., Zhou J., Liu X., Song Y., Xu H. E., Yi W. Chem. Commun. 2015;51:2925. - PubMed
- Huang J.-R., Qin L., Zhu Y.-Q., Song Q., Dong L. Chem. Commun. 2015;51:2844. - PubMed
-
-
-
For RhIII-catalyzed aromatic homologation via C–H coupling with two alkyne molecules: . See also: . For an example involving an initial transmetalation from a boronic acid derivative:
- Umeda N., Tsurugi H., Satoh T., Miura M. Angew. Chem., Int. Ed. 2008;47:4019. - PubMed
- Song G., Chen D., Pan C.-L., Crabtree R. H., Li X. J. Org. Chem. 2010;75:7487. - PubMed
- Mochida S., Shimizu M., Hirano K., Satoh T., Miura M. Chem.–Asian J. 2010;5:847. - PubMed
- Umeda N., Hirano K., Satoh T., Shibata N., Sato H., Miura M. J. Org. Chem. 2011;76:13. - PubMed
- Song G., Gong X., Li X. J. Org. Chem. 2011;76:7583. - PubMed
- Fukutani T., Hirano K., Satoh T., Miura M. J. Org. Chem. 2011;76:2867. - PubMed
- Shi Z., Tang C., Jiao N. Adv. Synth. Catal. 2012;354:2695.
- Li B., Ma J., Liang Y., Wang N., Xu S., Song H., Wang B. Eur. J. Org. Chem. 2013:1950.
- Zhao D., Nimphius C., Lindale M., Glorius F. Org. Lett. 2013;15:4504. - PubMed
- Nobushige K., Hirano K., Satoh T., Miura M. Org. Lett. 2014;16:1188. - PubMed
- Pham M. V., Cramer N. Angew. Chem., Int. Ed. 2014;53:3484. - PubMed
- Liu B., Hu F., Shi B.-F. Adv. Synth. Catal. 2014;356:2688.
- Wang H., Wang Y., Yang H., Tan C., Jiang Y., Zhao Y., Fu H. Adv. Synth. Catal. 2015;357:489.
- Wang J., Qin D., Lan J., Cheng Y., Zhang S., Guo Q., Wu J., Wu D., You J. Chem. Commun. 2015;51:6337. - PubMed
- Wang H., Wang Y., Yang H., Tan C., Jiang Y., Zhao Y., Fu H. Adv. Synth. Catal. 2015;357:489.
- Fukutani T., Hirano K., Satoh T., Miura M. Org. Lett. 2009;11:5198. - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources

