A five-coordinate Mn(iv) intermediate in biological water oxidation: spectroscopic signature and a pivot mechanism for water binding
- PMID: 29861966
- PMCID: PMC5950799
- DOI: 10.1039/c5sc03124a
A five-coordinate Mn(iv) intermediate in biological water oxidation: spectroscopic signature and a pivot mechanism for water binding
Abstract
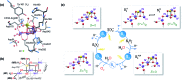
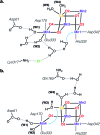
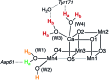
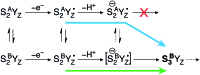
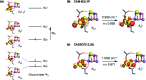
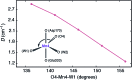
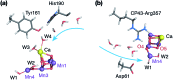
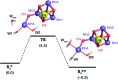
Among the four photo-driven transitions of the water-oxidizing tetramanganese-calcium cofactor of biological photosynthesis, the second-last step of the catalytic cycle, that is the S2 to S3 state transition, is the crucial step that poises the catalyst for the final O-O bond formation. This transition, whose intermediates are not yet fully understood, is a multi-step process that involves the redox-active tyrosine residue and includes oxidation and deprotonation of the catalytic cluster, as well as the binding of a water molecule. Spectroscopic data has the potential to shed light on the sequence of events that comprise this catalytic step, which still lacks a structural interpretation. In this work the S2-S3 state transition is studied and a key intermediate species is characterized: it contains a Mn3O4Ca cubane subunit linked to a five-coordinate Mn(iv) ion that adopts an approximately trigonal bipyramidal ligand field. It is shown using high-level density functional and multireference wave function calculations that this species accounts for the near-infrared absorption and electron paramagnetic resonance observations on metastable S2-S3 intermediates. The results confirm that deprotonation and Mn oxidation of the cofactor must precede the coordination of a water molecule, and lead to identification of a novel low-energy water binding mode that has important implications for the identity of the substrates in the mechanism of biological water oxidation.
Figures
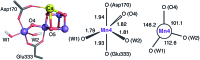
References
-
- Blankenship R. E., Molecular Mechanisms of Photosynthesis, Blackwell, Oxford, 2001.
-
- McEvoy J. P., Brudvig G. W. Chem. Rev. 2006;106:4455–4483. - PubMed
-
- Krewald V., Retegan M., Pantazis D. A. Top. Curr. Chem. 2016;371:23–48. - PubMed
-
- Cox N., Pantazis D. A., Neese F., Lubitz W. Acc. Chem. Res. 2013;46:1588–1596. - PubMed
-
- Dau H., Haumann M. Coord. Chem. Rev. 2008;252:273–295.
LinkOut - more resources
Full Text Sources
Other Literature Sources

