A straightforward method for automated Fmoc-based synthesis of bio-inspired peptide crypto-thioesters
- PMID: 29861986
- PMCID: PMC5952550
- DOI: 10.1039/c5sc02630j
A straightforward method for automated Fmoc-based synthesis of bio-inspired peptide crypto-thioesters
Abstract
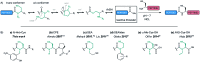
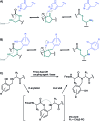
Despite recent advances, the direct Fmoc-based solid phase synthesis of peptide α-thioesters for the convergent synthesis of proteins via native chemical ligation (NCL) remains a challenge in the field. We herein report a simple and general methodology, enabling access to peptide thioester surrogates. A novel C-terminal N-(2-hydroxybenzyl)cysteine thioesterification device based on an amide-to-thioester rearrangement was developed, and the resulting peptide crypto-thioesters can be directly used in NCL reactions with fast N → S shift kinetics at neutral pH. These fast kinetics arise from our bio-inspired design, via intein-like intramolecular catalysis. Due to a well-positioned phenol moiety, an impressive >50 fold increase in the kinetic rate is observed compared to an O-methylated derivative. Importantly, the synthesis of this new device can be fully automated using inexpensive commercially available materials and does not require any post-synthetic steps prior to NCL. We successfully applied this new method to the synthesis of two long naturally-occurring cysteine-rich peptide sequences.
Figures


References
-
- Dawson P. E., Muir T. W., Clark-Lewis I., Kent S. B. Science. 1994;266:776–779. - PubMed
-
- Mende F., Seitz O. Angew. Chem., Int. Ed. 2011;50:1232–1240. - PubMed
-
- Fang G.-M., Li Y.-M., Shen F., Huang Y.-C., Li J.-B., Lin Y., Cui H.-K., Liu L. Angew. Chem., Int. Ed. 2011;50:7645–7649. - PubMed
-
- For side reactions observed with the Nbz approach see: Mahto S. K., Howard C. J., Shimko J. C., Ottesen J. J., ChemBioChem, 2011, 12 , 2488 –2494 , . Note that an article about a second generation Nbz linker was published during the preparation of this manuscript: Blanco-Canosa J. B., Nardone B., Albericio F., Dawson P. E., J. Am. Chem. Soc., 2015, 137 , 7197, –7209 , . For side reactions observed with the hydrazide approach, see: Mong S. K., Vinogradov A. A., Simon M. D., Pentelute B. L., ChemBioChem, 2014, 15 , 721 –733 . - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

