Photocatalytic proton reduction with ruthenium and cobalt complexes immobilized on fumed reversed-phase silica
- PMID: 29861992
- PMCID: PMC5952309
- DOI: 10.1039/c5sc02124c
Photocatalytic proton reduction with ruthenium and cobalt complexes immobilized on fumed reversed-phase silica
Abstract
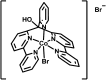
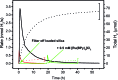
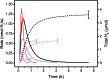
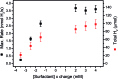
Heterogeneous photocatalytic hydrogen production with a non-covalently immobilized molecular ruthenium based photosensitizer (PS) and a cobalt polypyridyl based water reducing catalyst (WRC) is reported. PS and WRC were derivatized with C18-alkyl chains and immobilized by adsorption on hydrophobic fumed silica. The resulting loaded support was suspended in water with anionic or cationic surfactants and subjected to heterogeneous photocatalytic H2 production with ascorbate as sacrificial electron donor (SED). No leaching was observed under catalytic conditions, thus catalysis was truly heterogeneous. The catalytic performance of immobilized PS and WRC clearly exceeded that of homogeneous catalysis at low concentrations. At high concentration, diffusion and light limitation lead to lower reaction rates, but the same stability as for homogeneous reactions was still achieved. WRC concentration variations indicated a relatively high stability (up to 1300 H2/Co) and mobility of amphiphilic catalysts on the hydrophobic silica surface. Comparison of fumed silica with porous and non-porous silica showed, that a high BET surface area along with a good accessibility from the reaction media are crucial for catalytic performance. Mechanistic investigations by transient absorption spectroscopy displayed reductive quenching of excited PS by ascorbate followed by on particle electron transfer to WRC as reaction pathway. Particles with additional cationic surfactants exhibited a significantly higher catalytic performance as compared to anionic surfactants. Non-covalent anchoring of correspondingly derivatized WRCs or PSs to reversed-phase silica offers a rapid and versatile transition from homogeneous to heterogeneous molecular proton reduction.
Figures






References
-
- Styring S. Faraday Discuss. 2012;155:357–376. - PubMed
-
- Tachibana Y., Vayssieres L., Durrant J. R. Nat. Photonics. 2012;6:511–518.
-
- Dasgupta S., Brunschwig B. S., Winkler J. R., Gray H. B. Chem. Soc. Rev. 2013;42:2213–2214. - PubMed
-
- Cowan A. J., Durrant J. R. Chem. Soc. Rev. 2013;42:2281–2293. - PubMed
-
- Luo J., Im J. H., Mayer M. T., Schreier M., Khaja Nazeeruddin M., Park N.-G., Tilley S. D., Jin Fan H., Grätzel M. Science. 2014;345:1593–1596. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

