Protein Based Nanostructures for Drug Delivery
- PMID: 29862118
- PMCID: PMC5976961
- DOI: 10.1155/2018/9285854
Protein Based Nanostructures for Drug Delivery
Abstract
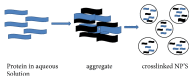
The key role of protein based nanostructures has recently revolutionized the nanomedicine era. Protein nanoparticles have turned out to be the major grounds for the transformation of different properties of many conventional materials by virtue of their size and greater surface area which instigates them to be more reactive to some other molecules. Protein nanoparticles have better biocompatibilities and biodegradability and also have the possibilities for surface modifications. These nanostructures can be synthesized by using protein like albumin, gelatin, whey protein, gliadin, legumin, elastin, zein, soy protein, and milk protein. The techniques for their fabrication include emulsification, desolvation, complex coacervation, and electrospray. The characterization parameters of protein nanoparticles comprise particle size, particle morphology, surface charge, drug loading, determination of drug entrapment, and particle structure and in vitro drug release. A plethora of protein nanoparticles applications via different routes of administration are explored and reported by eminent researchers which are highlighted in the present review along with the patents granted for protein nanoparticles as drug delivery carriers.
Figures

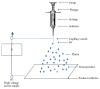

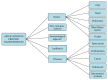
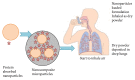
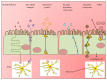
References
-
- Verma R. K., Garg S. Current status of drug delivery technologies and future directions. 2001;25:1–14.
-
- Pathak Y., Thassu D. Vol. 191. New York, NY, USA: Informa Healthcare; 2009. (Desgin nad formulation of protein based NPDDS).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

