LUBAC and ABIN-1 Modulate TRAIL-Based NF-κB Induction in Human Embryonic Kidney 293 Cells
- PMID: 29862142
- PMCID: PMC5982153
- DOI: 10.1089/biores.2018.0006
LUBAC and ABIN-1 Modulate TRAIL-Based NF-κB Induction in Human Embryonic Kidney 293 Cells
Abstract
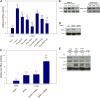
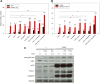
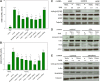
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is known to activate the canonical NF-κB pathway similar to TNF. The exact mechanism of the entire signaling cascade is still under investigation. The involvement of linear ubiquitylation as upregulating component has already been shown recently in some cell lines, but not in human embryonic kidney 293 (HEK293) cells. The downregulating function of the ABIN-1 (A20 binding and inhibitor of NF-κB) as linear ubiquitylation antagonist has been shown in combination with some NF-κB-inducing pathways, but not with TRAIL. We performed luciferase and western blot assays using HEK293 cells stimulated with either TRAIL (or TNF as a control) to analyze the involvement of linear ubiquitin chain assembly complex (LUBAC) components and the impact of ABIN-1 and ABIN-1-MAD (truncated form without A20 binding site) on NF-κB signaling. For overexpression experiments, we added plasmids of ABIN-1 and ABIN-1-MAD or LUBAC components HOIP, HOIL-1, or SHARPIN (single and combinations). For downregulation experiments five pairs of either SHARPIN, HOIL-1, or HOIP targeting miRNAs or one miRNA for ABIN-1 were designed and added. ABIN-1 and its truncated form ABIN-1-MAD reduced the NF-κB induction significantly indicating its involvement as antagonist (independent of deubiquitinase A20) of linear ubiquitylation in TRAIL-induced NF-κB signaling. In opposition, knockdown of ABIN-1 using a specific ABIN-1 miRNA led a clear increase of NF-κB signaling. Addition of single LUBAC components or combinations (except for SHARPIN with HOIL-1) resulted in clearly stronger NF-κB inductions. MiRNAs targeting LUBAC components significantly reduced NF-κB activation. Thus, in HEK293 cells linear ubiquitylation by LUBAC critically upregulates and ABIN-1 downregulates TRAIL-induced NF-κB signaling and may be interesting targets for future pathological therapies.
Keywords: ABIN-1; HEK 293 cells; LUBAC; NF-κB regulation; TRAIL.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
References
-
- Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol. 2011;12:439–452 - PubMed
-
- Kim KW, Kim BJ, Chung CW, et al. . Caspase cleavage product lacking amino-terminus of IkappaBalpha sensitizes resistant cells to TNF-alpha and TRAIL-induced apoptosis. J Cell Biochem. 2002;85:334–345 - PubMed
-
- Rothe M, Sarma V, Dixit VM, et al. . TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–1427 - PubMed
-
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
