Late-Stage Isotopic Carbon Labeling of Pharmaceutically Relevant Cyclic Ureas Directly from CO2
- PMID: 29862657
- PMCID: PMC6099343
- DOI: 10.1002/anie.201804838
Late-Stage Isotopic Carbon Labeling of Pharmaceutically Relevant Cyclic Ureas Directly from CO2
Abstract
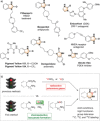
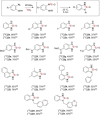
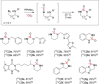
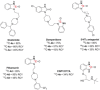
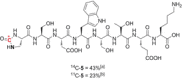
A robust, click-chemistry-inspired procedure for radiolabeling of cyclic ureas was developed. This protocol, suitable for all carbon isotopes (11 C, 13 C, 14 C), is based on the direct functionalization of carbon dioxide: the universal building block for carbon radiolabeling. The strategy is operationally simple and reproducible in different radiochemistry centers, exhibits remarkably wide substrate scope with short reaction times, and demonstrates superior reactivity as compared to previously reported systems. With this procedure, a variety of pharmaceuticals and an unprotected peptide were labeled with high radiochemical efficiency.
Keywords: carbon dioxide; carbon-11; carbon-14; heterocycles; isotopic labeling.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
[11C]CO2 BOP fixation with amines to access 11C-labeled ureas for PET imaging.J Labelled Comp Radiopharm. 2024 May 30;67(6):201-210. doi: 10.1002/jlcr.4075. Epub 2023 Dec 10. J Labelled Comp Radiopharm. 2024. PMID: 38073118
-
Dynamic Carbon Isotope Exchange of Pharmaceuticals with Labeled CO2.J Am Chem Soc. 2019 Jan 16;141(2):780-784. doi: 10.1021/jacs.8b12140. Epub 2018 Dec 31. J Am Chem Soc. 2019. PMID: 30586301
-
Synthesis of Ureas from CO2.Top Curr Chem (Cham). 2017 Apr;375(2):49. doi: 10.1007/s41061-017-0137-4. Epub 2017 Apr 10. Top Curr Chem (Cham). 2017. PMID: 28397187 Review.
-
Rapid and efficient synthesis of [11C]ureas via the incorporation of [11C]CO2 into aliphatic and aromatic amines.Chem Commun (Camb). 2013 Sep 25;49(74):8193-5. doi: 10.1039/c3cc44046j. Chem Commun (Camb). 2013. PMID: 23925577
-
Recent developments in carbonylation chemistry using [13 C]CO, [11 C]CO, and [14 C]CO.J Labelled Comp Radiopharm. 2018 Nov;61(13):949-987. doi: 10.1002/jlcr.3645. Epub 2018 Jun 28. J Labelled Comp Radiopharm. 2018. PMID: 29858516 Review.
Cited by
-
A Bifunctional Ionic Liquid for Capture and Electrochemical Conversion of CO2 to CO over Silver.ACS Catal. 2023 May 25;13(12):7812-7821. doi: 10.1021/acscatal.3c01538. eCollection 2023 Jun 16. ACS Catal. 2023. PMID: 37342831 Free PMC article.
-
Electrocatalytic Reactions for Converting CO2 to Value-Added Products: Recent Progress and Emerging Trends.Int J Mol Sci. 2023 Jun 9;24(12):9952. doi: 10.3390/ijms24129952. Int J Mol Sci. 2023. PMID: 37373100 Free PMC article. Review.
-
Copper-Catalyzed Carbonylative Cyclization of CO2: A Promising Approach for Synthesis of Flavone.Adv Sci (Weinh). 2025 Apr;12(13):e2415795. doi: 10.1002/advs.202415795. Epub 2025 Feb 7. Adv Sci (Weinh). 2025. PMID: 39921264 Free PMC article.
-
Recent Developments in Carbon-11 Chemistry and Applications for First-In-Human PET Studies.Molecules. 2023 Jan 17;28(3):931. doi: 10.3390/molecules28030931. Molecules. 2023. PMID: 36770596 Free PMC article. Review.
-
Monitoring In Vivo Performances of Protein-Drug Conjugates Using Site-Selective Dual Radiolabeling and Ex Vivo Digital Imaging.J Med Chem. 2022 May 12;65(9):6953-6968. doi: 10.1021/acs.jmedchem.2c00401. Epub 2022 May 2. J Med Chem. 2022. PMID: 35500280 Free PMC article.
References
-
- None
-
- Isin E. M., Elmore C. S., Nilsson G. N., Thompson R. A., Weidolf L., Chem. Res. Toxicol. 2012, 25, 532–542; - PubMed
-
- Penner N., Xu L., Prakash C., Chem. Res. Toxicol. 2012, 25, 513–531; - PubMed
-
- Atzrodt J., Derdau V., Kerr W. J., Reid M., Angew. Chem. Int. Ed. 2018, 57, 1758–1784; - PubMed
- Angew. Chem. 2018, 130, 1774–1802.
-
- None
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

