Chaihu-Shugan-San exerts an antidepressive effect by downregulating miR-124 and releasing inhibition of the MAPK14 and Gria3 signaling pathways
- PMID: 29863014
- PMCID: PMC5998613
- DOI: 10.4103/1673-5374.232478
Chaihu-Shugan-San exerts an antidepressive effect by downregulating miR-124 and releasing inhibition of the MAPK14 and Gria3 signaling pathways
Abstract
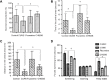
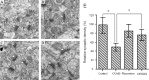
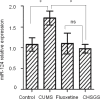
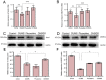
Dysregulation of miR-124 has been reported to be involved in the pathophysiology of depression. Chaihu-Shugan-San, a traditional Chinese medicine, has antidepressive activity; however, the underlying mechanisms remain unclear. In this study, to generate a rodent model of depression, rats were subjected to a combination of solitary confinement and chronic unpredictable mild stress for 28 days. Rats were intragastrically administered Chaihu-Shugan-San (2.835 mL/kg/d) for 4 weeks, once a day. Real-time reverse-transcription quantitative polymerase chain reaction, miRNA microarray, western blot assay and transmission electron microscopy demonstrated that Chaihu-Shugan-San downregulated miR-124 expression and upregulated the mRNA and protein levels of mitogen-activated protein kinase 14 (MAPK14) and glutamate receptor subunit 3 (Gria3). Chaihu-Shugan-San also promoted synapse formation in the hippocampus. The open field test, sucrose consumption test and forced swimming test were used to assess depression-like behavior. After intragastric administration of Chaihu-Shugan-San, sucrose consumption increased, while the depressive behaviors were substantially reduced. Together, these findings suggest that Chaihu-Shugan-San exerts an antidepressant-like effect by downregulating miR-124 expression and by releasing the inhibition of the MAPK14 and Gria3 signaling pathways.
Keywords: Chaihu-Shugan-San; Gria3; MAPK14; depression; forced swimming test; miR-124; nerve regeneration; neural plasticity; neural regeneration; open-field test; sucrose consumption test; traditional Chinese medicine.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Figures
References
-
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. - PubMed
-
- Belzeaux R, Bergon A, Jeanjean V, Loriod B, Formisano-Tréziny C, Verrier L, Loundou A, Baumstarck-Barrau K, Boyer L, Gall V, Gabert J, Nguyen C, Azorin JM, Naudin J, Ibrahim EC. Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl Psychiatry. 2012;2:e185. - PMC - PubMed
-
- Blier P. Neurobiology of depression and mechanism of action of depression treatments. J Clin Psychiatry. 2016;77:e319. - PubMed
-
- Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D, Corrada D, Milanesi L, Gennarelli M. Blood microRNA changes in depressed patients during antidepressant treatment. Eur Neuropsychopharmacol. 2013;23:602–611. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

