Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer
- PMID: 29863451
- PMCID: PMC6193457
- DOI: 10.1056/NEJMoa1803164
Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer
Abstract
Background: In the Suppression of Ovarian Function Trial (SOFT) and the Tamoxifen and Exemestane Trial (TEXT), the 5-year rates of recurrence of breast cancer were significantly lower among premenopausal women who received the aromatase inhibitor exemestane plus ovarian suppression than among those who received tamoxifen plus ovarian suppression. The addition of ovarian suppression to tamoxifen did not result in significantly lower recurrence rates than those with tamoxifen alone. Here, we report the updated results from the two trials.
Methods: Premenopausal women were randomly assigned to receive 5 years of tamoxifen, tamoxifen plus ovarian suppression, or exemestane plus ovarian suppression in SOFT and to receive tamoxifen plus ovarian suppression or exemestane plus ovarian suppression in TEXT. Randomization was stratified according to the receipt of chemotherapy.
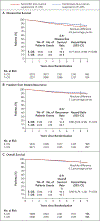
Results: In SOFT, the 8-year disease-free survival rate was 78.9% with tamoxifen alone, 83.2% with tamoxifen plus ovarian suppression, and 85.9% with exemestane plus ovarian suppression (P=0.009 for tamoxifen alone vs. tamoxifen plus ovarian suppression). The 8-year rate of overall survival was 91.5% with tamoxifen alone, 93.3% with tamoxifen plus ovarian suppression, and 92.1% with exemestane plus ovarian suppression (P=0.01 for tamoxifen alone vs. tamoxifen plus ovarian suppression); among the women who remained premenopausal after chemotherapy, the rates were 85.1%, 89.4%, and 87.2%, respectively. Among the women with cancers that were negative for HER2 who received chemotherapy, the 8-year rate of distant recurrence with exemestane plus ovarian suppression was lower than the rate with tamoxifen plus ovarian suppression (by 7.0 percentage points in SOFT and by 5.0 percentage points in TEXT). Grade 3 or higher adverse events were reported in 24.6% of the tamoxifen-alone group, 31.0% of the tamoxifen-ovarian suppression group, and 32.3% of the exemestane-ovarian suppression group.
Conclusions: Among premenopausal women with breast cancer, the addition of ovarian suppression to tamoxifen resulted in significantly higher 8-year rates of both disease-free and overall survival than tamoxifen alone. The use of exemestane plus ovarian suppression resulted in even higher rates of freedom from recurrence. The frequency of adverse events was higher in the two groups that received ovarian suppression than in the tamoxifen-alone group. (Funded by Pfizer and others; SOFT and TEXT ClinicalTrials.gov numbers, NCT00066690 and NCT00066703 , respectively.).
Figures




Comment in
-
Endocrine Adjuvant Therapy for Localized Breast Cancer.N Engl J Med. 2018 Jul 12;379(2):193-194. doi: 10.1056/NEJMe1806130. Epub 2018 Jun 4. N Engl J Med. 2018. PMID: 29863436 No abstract available.
-
Avoiding overuse of adjuvant therapies.Nat Rev Clin Oncol. 2018 Aug;15(8):469. doi: 10.1038/s41571-018-0054-7. Nat Rev Clin Oncol. 2018. PMID: 29921846 No abstract available.
-
Adjuvant Endocrine Therapy for Premenopausal Breast Cancer.N Engl J Med. 2018 Oct 25;379(17):1683. doi: 10.1056/NEJMc1810514. N Engl J Med. 2018. PMID: 30358970 No abstract available.
-
Adjuvant Endocrine Therapy for Premenopausal Breast Cancer.N Engl J Med. 2018 Oct 25;379(17):1683-4. doi: 10.1056/NEJMc1810514. N Engl J Med. 2018. PMID: 30358971 No abstract available.
-
Adjuvant Endocrine Therapy for Premenopausal Breast Cancer.N Engl J Med. 2018 Oct 25;379(17):1684. doi: 10.1056/NEJMc1810514. N Engl J Med. 2018. PMID: 30358972 No abstract available.
-
Living longer and living better: breast cancer endocrine therapy.BMJ Support Palliat Care. 2019 Sep;9(3):361-362. doi: 10.1136/bmjspcare-2018-001666. Epub 2019 Jan 18. BMJ Support Palliat Care. 2019. PMID: 30659045 No abstract available.
References
-
- LHRH-agonists in Early Breast Cancer Overview Goup. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet 2007; 369: 1711–23. - PubMed
-
- Aebi S, Gelber S, Castiglione-Gertsch M, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet 2000; 355: 1869–74. - PubMed
-
- Goldhirsch A, Gelber RD, Yothers G, et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr 2001; 30:4 4–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
