Resuscitation of Endotheliopathy and Bleeding in Thoracic Aortic Dissections: The VIPER-OCTA Randomized Clinical Pilot Trial
- PMID: 29863610
- PMCID: PMC6135474
- DOI: 10.1213/ANE.0000000000003545
Resuscitation of Endotheliopathy and Bleeding in Thoracic Aortic Dissections: The VIPER-OCTA Randomized Clinical Pilot Trial
Abstract
Background: Thoracic aorta dissection is an acute critical condition associated with shock-induced endotheliopathy, coagulopathy, massive bleeding, and significant morbidity and mortality. Our aim was to compare the effect of coagulation support with solvent/detergent-treated pooled plasma (OctaplasLG) versus standard fresh frozen plasma (FFP) on glycocalyx and endothelial injury, bleeding, and transfusion requirements.
Methods: Investigator-initiated, single-center, blinded, randomized clinical pilot trial of adult patients undergoing emergency surgery for thoracic aorta dissection. Patients were randomized to receive OctaplasLG or standard FFP as coagulation factor replacement related to bleeding. The primary outcome was glycocalyx and endothelial injury. Other outcomes included bleeding, transfusions and prohemostatics at 24 hours, organ failure, length of stay in the intensive care unit and in the hospital, safety, and mortality at 30 and 90 days.
Results: Fifty-seven patients were included to obtain 44 evaluable on the primary outcome. The OctaplasLG group displayed significantly reduced damage to the endothelial glycocalyx (syndecan-1) and reduced endothelial tight junction injury (sVE-cadherin) compared to standard FFP. In the OctaplasLG group compared to the standard FFP, days on ventilator (1 day [interquartile range, 0-1] vs 2 days [1-3]; P = .013), bleeding during surgery (2150 [1600-3087] vs 2750 [2130-6875]; P = .046), 24-hour total transfusion and platelet transfusion volume (3975 mL [2640-6828 mL] vs 6220 mL [4210-10,245 mL]; P = .040, and 1400 mL [1050-2625 mL] vs 2450 mL [1400-3500 mL]; P = .027), and goal-directed use of prohemostatics (7/23 [30.4%] vs 13/21 [61.9%]; P = .036) were all significantly lower. Among the 57 patients randomized, 30-day mortality was 20.7% (6/29) in the OctaplasLG group and 25% (7/28) in the standard FFP group (P = .760). No safety concern was raised.
Conclusions: In this randomized, clinical pilot trial of patients undergoing emergency surgery for thoracic aorta dissections, we found that OctaplasLG reduced glycocalyx and endothelial injury, reduced bleeding, transfusions, use of prohemostatics, and time on ventilator after surgery compared to standard FFP. An adequately powered multicenter trial is warranted to confirm the clinical importance of the findings.
Trial registration: ClinicalTrials.gov NCT02253082.
Conflict of interest statement
Conflicts of Interest: See Disclosures at the end of the article.
Figures
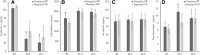
Similar articles
-
Fresh frozen plasma (FFP) use during massive blood transfusion in trauma resuscitation.Injury. 2010 Jan;41(1):35-9. doi: 10.1016/j.injury.2009.09.029. Injury. 2010. PMID: 19833331
-
Plasma as endothelial rescue in septic shock: A randomized, phase 2a pilot trial.Transfusion. 2024 Sep;64(9):1653-1661. doi: 10.1111/trf.17939. Epub 2024 Jul 8. Transfusion. 2024. PMID: 38973502 Clinical Trial.
-
Resuscitation with Pooled and Pathogen-Reduced Plasma Attenuates the Increase in Brain Water Content following Traumatic Brain Injury and Hemorrhagic Shock in Rats.J Neurotrauma. 2017 Mar 1;34(5):1054-1062. doi: 10.1089/neu.2016.4574. Epub 2016 Oct 13. J Neurotrauma. 2017. PMID: 27626366
-
Fresh frozen plasma and platelet transfusion for nonbleeding patients in the intensive care unit: benefit or harm?Crit Care Med. 2006 May;34(5 Suppl):S170-3. doi: 10.1097/01.CCM.0000214288.88308.26. Crit Care Med. 2006. PMID: 16617262 Review.
-
[Endovascular interventions of the descending thoracic aorta].Herz. 2006 Aug;31(5):429-33. doi: 10.1007/s00059-006-2838-2. Herz. 2006. PMID: 16944062 Review. German.
Cited by
-
Plasma as a resuscitation fluid for volume-depleted shock: Potential benefits and risks.Transfusion. 2021 Jul;61 Suppl 1(Suppl 1):S301-S312. doi: 10.1111/trf.16462. Epub 2021 May 31. Transfusion. 2021. PMID: 34057210 Free PMC article. Review. No abstract available.
-
Fibrinogen inhibits microRNA-19b, a novel mechanism for repair of haemorrhagic shock-induced endothelial cell dysfunction.Blood Transfus. 2021 Sep;19(5):420-427. doi: 10.2450/2021.0361-20. Epub 2021 Jan 27. Blood Transfus. 2021. PMID: 33539284 Free PMC article.
-
Resuscitative Strategies to Modulate the Endotheliopathy of Trauma: From Cell to Patient.Shock. 2020 May;53(5):575-584. doi: 10.1097/SHK.0000000000001378. Shock. 2020. PMID: 31090680 Free PMC article. Review.
-
Proceedings of the Food and Drug Administration public workshop on pathogen reduction technologies for blood safety 2018 (Commentary, p. 3026).Transfusion. 2019 Sep;59(9):3002-3025. doi: 10.1111/trf.15344. Epub 2019 May 29. Transfusion. 2019. PMID: 31144334 Free PMC article. No abstract available.
-
Intraoperative Hemostatic Agents in Thoracic Aortic Surgery-A Scoping Review.J Clin Med. 2025 Jun 5;14(11):4001. doi: 10.3390/jcm14114001. J Clin Med. 2025. PMID: 40507762 Free PMC article. Review.
References
-
- Sigman MM, Palmer OP, Ham SW, Cunningham M, Weaver FA. Aortic morphologic findings after thoracic endovascular aortic repair for type B aortic dissection. JAMA Surg. 2014;149:977–983.. - PubMed
-
- Mussa FF, Horton JD, Moridzadeh R, Nicholson J, Trimarchi S, Eagle KA. Acute aortic dissection and intramural hematoma: a systematic review. JAMA. 2016;316:754–763.. - PubMed
-
- LeMaire SA, Russell L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011;8:103–113.. - PubMed
-
- Hiratzka LF, Bakris GL, Beckman JA, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369.. - PubMed
-
- Rehm M, Bruegger D, Christ F, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906.. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

