Enrichment of Bacterial Lipoproteins and Preparation of N-terminal Lipopeptides for Structural Determination by Mass Spectrometry
- PMID: 29863685
- PMCID: PMC6101299
- DOI: 10.3791/56842
Enrichment of Bacterial Lipoproteins and Preparation of N-terminal Lipopeptides for Structural Determination by Mass Spectrometry
Abstract
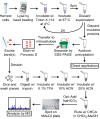
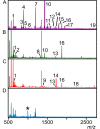
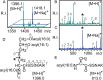
Lipoproteins are important constituents of the bacterial cell envelope and potent activators of the mammalian innate immune response. Despite their significance to both cell physiology and immunology, much remains to be discovered about novel lipoprotein forms, how they are synthesized, and the effect of the various forms on host immunity. To enable thorough studies on lipoproteins, this protocol describes a method for bacterial lipoprotein enrichment and preparation of N-terminal tryptic lipopeptides for structural determination by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). Expanding on an established Triton X-114 phase partitioning method for lipoprotein extraction and enrichment from the bacterial cell membrane, the protocol includes additional steps to remove non-lipoprotein contaminants, increasing lipoprotein yield and purity. Since lipoproteins are commonly used in Toll-like receptor (TLR) assays, it is critical to first characterize the N-terminal structure by MALDI-TOF MS. Herein, a method is presented to isolate concentrated hydrophobic peptides enriched in N-terminal lipopeptides suitable for direct analysis by MALDI-TOF MS/MS. Lipoproteins that have been separated by Sodium Dodecyl Sulfate Poly-Acrylamide Gel Electrophoresis (SDS-PAGE) are transferred to a nitrocellulose membrane, digested in situ with trypsin, sequentially washed to remove polar tryptic peptides, and finally eluted with chloroform-methanol. When coupled with MS of the more polar trypsinized peptides from wash solutions, this method provides the ability to both identify the lipoprotein and characterize its N-terminus in a single experiment. Intentional sodium adduct formation can also be employed as a tool to promote more structurally informative fragmentation spectra. Ultimately, enrichment of lipoproteins and determination of their N-terminal structures will permit more extensive studies on this ubiquitous class of bacterial proteins.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases