Cardiac metabolic effects of KNa1.2 channel deletion and evidence for its mitochondrial localization
- PMID: 29863912
- PMCID: PMC6181635
- DOI: 10.1096/fj.201800139R
Cardiac metabolic effects of KNa1.2 channel deletion and evidence for its mitochondrial localization
Abstract
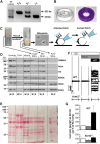
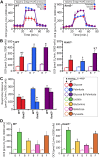
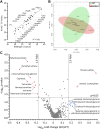
Controversy surrounds the molecular identity of mitochondrial K+ channels that are important for protection against cardiac ischemia-reperfusion injury. Although KNa1.2 (sodium-activated potassium channel encoded by Kcn2) is necessary for cardioprotection by volatile anesthetics, electrophysiological evidence for a channel of this type in mitochondria is lacking. The endogenous physiological role of a potential mito-KNa1.2 channel is also unclear. In this study, single channel patch-clamp of 27 independent cardiac mitochondrial inner membrane (mitoplast) preparations from wild-type (WT) mice yielded 6 channels matching the known ion sensitivity, ion selectivity, pharmacology, and conductance properties of KNa1.2 (slope conductance, 138 ± 1 pS). However, similar experiments on 40 preparations from Kcnt2-/- mice yielded no such channels. The KNa opener bithionol uncoupled respiration in WT but not Kcnt2-/- cardiomyocytes. Furthermore, when oxidizing only fat as substrate, Kcnt2-/- cardiomyocytes and hearts were less responsive to increases in energetic demand. Kcnt2-/- mice also had elevated body fat, but no baseline differences in the cardiac metabolome. These data support the existence of a cardiac mitochondrial KNa1.2 channel, and a role for cardiac KNa1.2 in regulating metabolism under conditions of high energetic demand.-Smith, C. O., Wang, Y. T., Nadtochiy, S. M., Miller, J. H., Jonas, E. A., Dirksen, R. T., Nehrke, K., Brookes, P. S. Cardiac metabolic effects of KNa1.2 channel deletion and evidence for its mitochondrial localization.
Keywords: Slick; Slo2.1; bithionol; patch clamp.
Conflict of interest statement
The authors thank Christopher Lingle (Washington University, St. Louis, MO, USA) for providing founders for the
Figures





Similar articles
-
The Epilepsy of Infancy With Migrating Focal Seizures: Identification of de novo Mutations of the KCNT2 Gene That Exert Inhibitory Effects on the Corresponding Heteromeric KNa1.1/KNa1.2 Potassium Channel.Front Cell Neurosci. 2020 Jan 24;14:1. doi: 10.3389/fncel.2020.00001. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32038177 Free PMC article.
-
Increased Expression of KNa1.2 Channel by MAPK Pathway Regulates Neuronal Activity Following Traumatic Brain Injury.Neurochem Res. 2024 Feb;49(2):427-440. doi: 10.1007/s11064-023-04044-1. Epub 2023 Oct 25. Neurochem Res. 2024. PMID: 37875713
-
Cardiac Slo2.1 Is Required for Volatile Anesthetic Stimulation of K+ Transport and Anesthetic Preconditioning.Anesthesiology. 2016 May;124(5):1065-76. doi: 10.1097/ALN.0000000000001046. Anesthesiology. 2016. PMID: 26845140 Free PMC article.
-
The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection.Biochem J. 2017 Jun 9;474(12):2067-2094. doi: 10.1042/BCJ20160623. Biochem J. 2017. PMID: 28600454 Free PMC article. Review.
-
[Molecular and functional diversity of ATP-sensitive K+ channels: the pathophysiological roles and potential drug targets].Nihon Yakurigaku Zasshi. 2003 Sep;122(3):243-50. doi: 10.1254/fpj.122.243. Nihon Yakurigaku Zasshi. 2003. PMID: 12939542 Review. Japanese.
Cited by
-
Modified Blue Native Gel Approach for Analysis of Respiratory Supercomplexes.Methods Mol Biol. 2021;2276:227-234. doi: 10.1007/978-1-0716-1266-8_17. Methods Mol Biol. 2021. PMID: 34060045 Free PMC article.
-
The Interplay between Dysregulated Ion Transport and Mitochondrial Architecture as a Dangerous Liaison in Cancer.Int J Mol Sci. 2021 May 14;22(10):5209. doi: 10.3390/ijms22105209. Int J Mol Sci. 2021. PMID: 34069047 Free PMC article. Review.
-
Discovery of the First Orally Available, Selective KNa1.1 Inhibitor: In Vitro and In Vivo Activity of an Oxadiazole Series.ACS Med Chem Lett. 2021 Mar 9;12(4):593-602. doi: 10.1021/acsmedchemlett.0c00675. eCollection 2021 Apr 8. ACS Med Chem Lett. 2021. PMID: 33859800 Free PMC article.
-
Lactate Dehydrogenase Inhibition With Oxamate Exerts Bone Anabolic Effect.J Bone Miner Res. 2020 Dec;35(12):2432-2443. doi: 10.1002/jbmr.4142. Epub 2020 Sep 19. J Bone Miner Res. 2020. PMID: 32729639 Free PMC article.
-
Methods of Measuring Mitochondrial Potassium Channels: A Critical Assessment.Int J Mol Sci. 2022 Jan 21;23(3):1210. doi: 10.3390/ijms23031210. Int J Mol Sci. 2022. PMID: 35163132 Free PMC article. Review.
References
-
- Bentzen B. H., Nardi A., Calloe K., Madsen L. S., Olesen S. P., Grunnet M. (2007) The small molecule NS11021 is a potent and specific activator of Ca2+-activated big-conductance K+ channels. Mol. Pharmacol. 72, 1033–1044 - PubMed
-
- Garlid K. D., Paucek P., Yarov-Yarovoy V., Murray H. N., Darbenzio R. B., D’Alonzo A. J., Lodge N. J., Smith M. A., Grover G. J. (1997) Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ. Res. 81, 1072–1082 - PubMed
-
- Inoue I., Nagase H., Kishi K., Higuti T. (1991) ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature 352, 244–247 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

