Translational repression by an RNA-binding protein promotes differentiation to infective forms in Trypanosoma cruzi
- PMID: 29864162
- PMCID: PMC6002132
- DOI: 10.1371/journal.ppat.1007059
Translational repression by an RNA-binding protein promotes differentiation to infective forms in Trypanosoma cruzi
Abstract
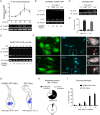
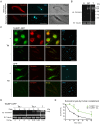
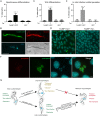
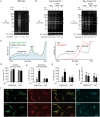
Trypanosomes, protozoan parasites of medical importance, essentially rely on post-transcriptional mechanisms to regulate gene expression in insect vectors and vertebrate hosts. RNA binding proteins (RBPs) that associate to the 3'-UTR of mature mRNAs are thought to orchestrate master developmental programs for these processes to happen. Yet, the molecular mechanisms by which differentiation occurs remain largely unexplored in these human pathogens. Here, we show that ectopic inducible expression of the RBP TcUBP1 promotes the beginning of the differentiation process from non-infective epimastigotes to infective metacyclic trypomastigotes in Trypanosoma cruzi. In early-log epimastigotes TcUBP1 promoted a drop-like phenotype, which is characterized by the presence of metacyclogenesis hallmarks, namely repositioning of the kinetoplast, the expression of an infective-stage virulence factor such as trans-sialidase, increased resistance to lysis by human complement and growth arrest. Furthermore, TcUBP1-ectopic expression in non-infective late-log epimastigotes promoted full development into metacyclic trypomastigotes. TcUBP1-derived metacyclic trypomastigotes were infective in cultured cells, and developed normally into amastigotes in the cytoplasm. By artificial in vivo tethering of TcUBP1 to the 3' untranslated region of a reporter mRNA we were able to determine that translation of the reporter was reduced by 8-fold, while its mRNA abundance was not significantly compromised. Inducible ectopic expression of TcUBP1 confirmed its role as a translational repressor, revealing significant reduction in the translation rate of multiple proteins, a reduction of polysomes, and promoting the formation of mRNA granules. Expression of TcUBP1 truncated forms revealed the requirement of both N and C-terminal glutamine-rich low complexity sequences for the development of the drop-like phenotype in early-log epimastigotes. We propose that a rise in TcUBP1 levels, in synchrony with nutritional deficiency, can promote the differentiation of T. cruzi epimastigotes into infective metacyclic trypomastigotes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Transcriptomic analysis of N-terminal mutated Trypanosoma cruzi UBP1 knockdown underlines the importance of this RNA-binding protein in parasite development.PLoS Negl Trop Dis. 2024 May 17;18(5):e0012179. doi: 10.1371/journal.pntd.0012179. eCollection 2024 May. PLoS Negl Trop Dis. 2024. PMID: 38758959 Free PMC article.
-
The RNA-binding protein TcUBP1 up-regulates an RNA regulon for a cell surface-associated Trypanosoma cruzi glycoprotein and promotes parasite infectivity.J Biol Chem. 2019 Jun 28;294(26):10349-10364. doi: 10.1074/jbc.RA118.007123. Epub 2019 May 21. J Biol Chem. 2019. PMID: 31113862 Free PMC article.
-
Knockout of the CCCH zinc finger protein TcZC3H31 blocks Trypanosoma cruzi differentiation into the infective metacyclic form.Mol Biochem Parasitol. 2018 Apr;221:1-9. doi: 10.1016/j.molbiopara.2018.01.006. Epub 2018 Feb 2. Mol Biochem Parasitol. 2018. PMID: 29409763
-
Genetic structure and expression of the surface glycoprotein GP82, the main adhesin of Trypanosoma cruzi metacyclic trypomastigotes.ScientificWorldJournal. 2013;2013:156734. doi: 10.1155/2013/156734. Epub 2013 Feb 4. ScientificWorldJournal. 2013. PMID: 23431251 Free PMC article. Review.
-
Trypanosoma cruzi infection by oral route: how the interplay between parasite and host components modulates infectivity.Parasitol Int. 2008 Jun;57(2):105-9. doi: 10.1016/j.parint.2007.12.008. Epub 2007 Dec 23. Parasitol Int. 2008. PMID: 18234547 Review.
Cited by
-
Metacyclogenesis as the Starting Point of Chagas Disease.Int J Mol Sci. 2023 Dec 21;25(1):117. doi: 10.3390/ijms25010117. Int J Mol Sci. 2023. PMID: 38203289 Free PMC article. Review.
-
GCN2-Like Kinase Modulates Stress Granule Formation During Nutritional Stress in Trypanosoma cruzi.Front Cell Infect Microbiol. 2020 Apr 16;10:149. doi: 10.3389/fcimb.2020.00149. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32373547 Free PMC article.
-
Transcriptomic analysis of N-terminal mutated Trypanosoma cruzi UBP1 knockdown underlines the importance of this RNA-binding protein in parasite development.PLoS Negl Trop Dis. 2024 May 17;18(5):e0012179. doi: 10.1371/journal.pntd.0012179. eCollection 2024 May. PLoS Negl Trop Dis. 2024. PMID: 38758959 Free PMC article.
-
An AMP-activated protein kinase complex with two distinctive alpha subunits is involved in nutritional stress responses in Trypanosoma cruzi.PLoS Negl Trop Dis. 2021 May 24;15(5):e0009435. doi: 10.1371/journal.pntd.0009435. eCollection 2021 May. PLoS Negl Trop Dis. 2021. PMID: 34029334 Free PMC article.
-
Comparative analysis of the transcriptional responses of five Leishmania species to trivalent antimony.Parasit Vectors. 2021 Aug 21;14(1):419. doi: 10.1186/s13071-021-04915-y. Parasit Vectors. 2021. PMID: 34419127 Free PMC article.
References
-
- Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell. 2009;136(4):688–700. doi: 10.1016/j.cell.2009.02.001 . - DOI - PubMed
-
- Clery A, Allain FH. From Structure to Function of RNA Binding Domains In: RNA Binding Proteins. 2012;Chapter 9:137–58.
-
- Calabretta S, Richard S. Emerging Roles of Disordered Sequences in RNA-Binding Proteins. Trends in biochemical sciences. 2015;40(11):662–72. doi: 10.1016/j.tibs.2015.08.012 . - DOI - PubMed
-
- Kolev NG, Ullu E, Tschudi C. The emerging role of RNA-binding proteins in the life cycle of Trypanosoma brucei. Cellular microbiology. 2014;16(4):482–9. doi: 10.1111/cmi.12268 - DOI - PMC - PubMed
-
- Clayton C, Shapira M. Post-transcriptional regulation of gene expression in trypanosomes and leishmanias. Molecular and biochemical parasitology. 2007;156(2):93–101. doi: 10.1016/j.molbiopara.2007.07.007 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

