Short- and long-term neonatal outcomes according to differential exposure to antenatal corticosteroid therapy in preterm births prior to 24 weeks of gestation
- PMID: 29864169
- PMCID: PMC5986118
- DOI: 10.1371/journal.pone.0198471
Short- and long-term neonatal outcomes according to differential exposure to antenatal corticosteroid therapy in preterm births prior to 24 weeks of gestation
Abstract
Aim: To assess the effects of differential exposure to antenatal corticosteroid (ACS) on short- and long-term outcomes of infants born before 24 weeks of gestation.
Methods: This is a retrospective cohort study of 147 infants delivered by 116 women at 21-23 weeks of gestation between January 2001 and December 2016 at a tertiary referral hospital in Seoul, Korea. Eligible subjects were categorized into the following three groups according to ACS exposure: non-user (n = 53), partial-course (n = 44), and complete-course (n = 50). Univariable and multivariable analyses were used to compare neonatal mortality, neonatal morbidities including intraventricular hemorrhage (IVH), and neurodevelopmental impairment including cerebral palsy among the three groups.
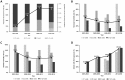
Results: Neonatal mortality rate was significantly lower in the ACS-user groups (non-user, 52.8%; partial-course, 27.3%; complete-course, 28.0%; P = 0.01), but complete-course of ACS therapy had no advantages over partial-course. A lower incidence of IVH was observed in the complete-course group (non-users, 54.8%; partial-course, 48.6%; complete-course, 20.5%; P = 0.003). Multiple logistic regression analysis showed that ACS therapy, either partial- or complete-course, was associated with a lower rate of neonatal mortality (adjusted odds ratio (aOR) 0.375; 95% confidence interval (CI) 0.141-0.996 in partial-course; aOR 0.173; 95% CI 0.052-0.574) in complete-course). IVH (aOR 0.191; 95% CI 0.071-0.516) was less likely to occur in the complete-course group than in the non-user group. Neurodevelopmental impairment of survivors at 18-22 month after birth was not significantly different among the three groups.
Conclusion: ACS therapy in preterm births at 21-23 weeks of gestation was associated with significantly reduced rates of neonatal mortality and IVH, especially with complete administration.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Association of Neurodevelopmental Outcomes and Neonatal Morbidities of Extremely Premature Infants With Differential Exposure to Antenatal Steroids.JAMA Pediatr. 2016 Dec 1;170(12):1164-1172. doi: 10.1001/jamapediatrics.2016.1936. JAMA Pediatr. 2016. PMID: 27723868 Free PMC article.
-
Efficacy of antenatal corticosteroids in preterm twins: the EPIPAGE-2 cohort study.BJOG. 2018 Aug;125(9):1164-1170. doi: 10.1111/1471-0528.15014. Epub 2017 Dec 14. BJOG. 2018. PMID: 29119673
-
Neurodevelopmental outcomes of preterm infants conceived by assisted reproductive technology.Am J Obstet Gynecol. 2021 Sep;225(3):276.e1-276.e9. doi: 10.1016/j.ajog.2021.03.027. Epub 2021 Mar 30. Am J Obstet Gynecol. 2021. PMID: 33798481
-
[Prevention of preterm birth complications by antenatal corticosteroid administration].J Gynecol Obstet Biol Reprod (Paris). 2016 Dec;45(10):1399-1417. doi: 10.1016/j.jgyn.2016.09.008. Epub 2016 Oct 21. J Gynecol Obstet Biol Reprod (Paris). 2016. PMID: 27776846 Review. French.
-
Head midline position for preventing the occurrence or extension of germinal matrix-intraventricular haemorrhage in preterm infants.Cochrane Database Syst Rev. 2020 Jul 7;7(7):CD012362. doi: 10.1002/14651858.CD012362.pub3. Cochrane Database Syst Rev. 2020. PMID: 32639053 Free PMC article.
Cited by
-
Evaluation of Long-term Outcomes Associated With Preterm Exposure to Antenatal Corticosteroids: A Systematic Review and Meta-analysis.JAMA Pediatr. 2022 Jun 1;176(6):e220483. doi: 10.1001/jamapediatrics.2022.0483. Epub 2022 Jun 6. JAMA Pediatr. 2022. PMID: 35404395 Free PMC article.
-
Increased Risk for Respiratory Complications in Male Extremely Preterm Infants: A Propensity Score Matching Study.Front Endocrinol (Lausanne). 2022 May 12;13:823707. doi: 10.3389/fendo.2022.823707. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35634508 Free PMC article.
-
Survival outcomes among periviable infants: a systematic review and meta-analysis comparing different income countries and time periods.Front Public Health. 2024 Dec 30;12:1454433. doi: 10.3389/fpubh.2024.1454433. eCollection 2024. Front Public Health. 2024. PMID: 39807383 Free PMC article.
References
-
- Obstetric Care Consensus No. 4: Periviable Birth. Obstetrics and gynecology. 2016;127(6):e157–69. Epub 2016/05/24. doi: 10.1097/AOG.0000000000001483 . - DOI - PubMed
-
- Crane J, Armson A, Brunner M, De La Ronde S, Farine D, Keenan-Lindsay L, et al. Antenatal corticosteroid therapy for fetal maturation. Journal of obstetrics and gynaecology Canada: JOGC = Journal d’obstetrique et gynecologie du Canada: JOGC. 2003;25(1):45–52. Epub 2003/01/28. . - PubMed
-
- Roberts D. Antenatal corticosteroids to reduce neonatal morbidity and mortality. Green-top Guideline No 7. 2010. - PubMed
-
- Antenatal Corticosteroids Clinical Practice Guidelines Panel Antenatal corticosteroids given to women prior to birth to improve fetal, infant,child and adult health: Clinical Practice Guidelines. Auckland, New Zealand: Liggins Institute, The University of Auckland; 2015.
-
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. The Cochrane database of systematic reviews. 2006;(3):Cd004454 Epub 2006/07/21. doi: 10.1002/14651858.CD004454.pub2 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

