Role of Amyloid-β and Tau Proteins in Alzheimer's Disease: Confuting the Amyloid Cascade
- PMID: 29865055
- PMCID: PMC8371153
- DOI: 10.3233/JAD-179935
Role of Amyloid-β and Tau Proteins in Alzheimer's Disease: Confuting the Amyloid Cascade
Erratum in
-
Role of Amyloid-β and Tau Proteins in Alzheimer's Disease: Confuting the Amyloid Cascade.J Alzheimers Dis. 2019;68(1):415. doi: 10.3233/JAD-189015. J Alzheimers Dis. 2019. PMID: 30883364 No abstract available.
Abstract
The "Amyloid Cascade Hypothesis" has dominated the Alzheimer's disease (AD) field in the last 25 years. It posits that the increase of amyloid-β (Aβ) is the key event in AD that triggers tau pathology followed by neuronal death and eventually, the disease. However, therapeutic approaches aimed at decreasing Aβ levels have so far failed, and tau-based clinical trials have not yet produced positive findings. This begs the question of whether the hypothesis is correct. Here we have examined literature on the role of Aβ and tau in synaptic dysfunction, memory loss, and seeding and spreading of AD, highlighting important parallelisms between the two proteins in all of these phenomena. We discuss novel findings showing binding of both Aβ and tau oligomers to amyloid-β protein precursor (AβPP), and the requirement for the presence of this protein for both Aβ and tau to enter neurons and induce abnormal synaptic function and memory. Most importantly, we propose a novel view of AD pathogenesis in which extracellular oligomers of Aβ and tau act in parallel and upstream of AβPP. Such a view will call for a reconsideration of therapeutic approaches directed against Aβ and tau, paving the way to an increased interest toward AβPP, both for understanding the pathogenesis of the disease and elaborating new therapeutic strategies.
Keywords: Amyloid-β peptide; amyloid-β protein precursor; oligomers; synaptic dysfunction; tau.
Figures
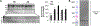
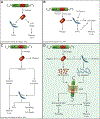
References
-
- Prince M, Wimo A, Guerchet M, Ali GC, Wu Y-T, Prina M, Chan KY, Xia Z (2015) World Alzheimer Report 2015. The global impact of dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer Disease International, London.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

