Aromaticity as a Guiding Concept for Spectroscopic Features and Nonlinear Optical Properties of Porphyrinoids
- PMID: 29865191
- PMCID: PMC6100263
- DOI: 10.3390/molecules23061333
Aromaticity as a Guiding Concept for Spectroscopic Features and Nonlinear Optical Properties of Porphyrinoids
Abstract
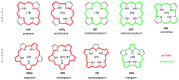
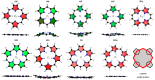
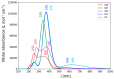
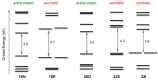
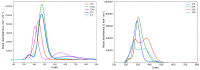
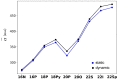
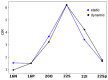
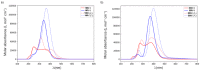
With their versatile molecular topology and aromaticity, porphyrinoid systems combine remarkable chemistry with interesting photophysical properties and nonlinear optical properties. Hence, the field of application of porphyrinoids is very broad ranging from near-infrared dyes to opto-electronic materials. From previous experimental studies, aromaticity emerges as an important concept in determining the photophysical properties and two-photon absorption cross sections of porphyrinoids. Despite a considerable number of studies on porphyrinoids, few investigate the relationship between aromaticity, UV/vis absorption spectra and nonlinear properties. To assess such structure-property relationships, we performed a computational study focusing on a series of Hückel porphyrinoids to: (i) assess their (anti)aromatic character; (ii) determine the fingerprints of aromaticity on the UV/vis spectra; (iii) evaluate the role of aromaticity on the NLO properties. Using an extensive set of aromaticity descriptors based on energetic, magnetic, structural, reactivity and electronic criteria, the aromaticity of [4n+2] π-electron porphyrinoids was evidenced as was the antiaromaticity for [4n] π-electron systems. In agreement with previous studies, the absorption spectra of aromatic systems display more intense B and Q bands in comparison to their antiaromatic homologues. The nature of these absorption bands was analyzed in detail in terms of polarization, intensity, splitting and composition. Finally, quantities such as the average polarizability and its anisotropy were found to be larger in aromatic systems, whereas first and second hyperpolarizability are influenced by the interplay between aromaticity, planarity and molecular symmetry. To conclude, aromaticity dictates the photophysical properties in porphyrinoids, whereas it is not the only factor determining the magnitude of NLO properties.
Keywords: absorption spectra; aromaticity; nonlinear optical properties; porphyrinoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Description of aromaticity in porphyrinoids.J Am Chem Soc. 2013 Jan 9;135(1):315-21. doi: 10.1021/ja309434t. Epub 2012 Dec 21. J Am Chem Soc. 2013. PMID: 23205604
-
Aromaticity in the Spectroscopic Spotlight of Hexaphyrins.Chemistry. 2024 Sep 16;30(52):e202401933. doi: 10.1002/chem.202401933. Epub 2024 Aug 12. Chemistry. 2024. PMID: 38889264
-
Control and Switching of Aromaticity in Various All-Aza-Expanded Porphyrins: Spectroscopic and Theoretical Analyses.Chem Rev. 2017 Feb 22;117(4):2257-2312. doi: 10.1021/acs.chemrev.6b00313. Epub 2016 Dec 16. Chem Rev. 2017. PMID: 27981841
-
Porphyrinoids, a unique platform for exploring excited-state aromaticity.Chem Soc Rev. 2022 Jan 4;51(1):268-292. doi: 10.1039/d1cs00742d. Chem Soc Rev. 2022. PMID: 34879124 Review.
-
Synthesis and Properties of Stable 20π Porphyrinoids.Chem Rec. 2022 Nov;22(11):e202200144. doi: 10.1002/tcr.202200144. Epub 2022 Jul 27. Chem Rec. 2022. PMID: 35896952 Review.
Cited by
-
Quest for the Most Aromatic Pathway in Charged Expanded Porphyrins.Chemistry. 2023 Jan 27;29(6):e202202264. doi: 10.1002/chem.202202264. Epub 2022 Nov 28. Chemistry. 2023. PMID: 36194440 Free PMC article.
-
A chromophore-supported structural and functional model of dinuclear copper enzymes, for facilitating mechanism of action studies.Chem Sci. 2021 Aug 10;12(37):12445-12450. doi: 10.1039/d1sc02593g. eCollection 2021 Sep 29. Chem Sci. 2021. PMID: 34603675 Free PMC article.
-
Biological Evaluation and Structural Analysis of Some Aminodiphenylamine Derivatives.Antioxidants (Basel). 2023 Mar 13;12(3):713. doi: 10.3390/antiox12030713. Antioxidants (Basel). 2023. PMID: 36978960 Free PMC article.
-
Reversible π-system switching of thiophene-fused thiahexaphyrins by solvent and oxidation/reduction.Chem Sci. 2018 Aug 14;9(38):7528-7539. doi: 10.1039/c8sc02448k. eCollection 2018 Oct 14. Chem Sci. 2018. PMID: 30319753 Free PMC article.
-
Fine-Tuning of Nonlinear Optical Contrasts of Hexaphyrin-Based Molecular Switches Using Inverse Design.Front Chem. 2021 Dec 3;9:786036. doi: 10.3389/fchem.2021.786036. eCollection 2021. Front Chem. 2021. PMID: 34926405 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

