Results of Beta Secretase-Inhibitor Clinical Trials Support Amyloid Precursor Protein-Independent Generation of Beta Amyloid in Sporadic Alzheimer's Disease
- PMID: 29865246
- PMCID: PMC6024788
- DOI: 10.3390/medsci6020045
Results of Beta Secretase-Inhibitor Clinical Trials Support Amyloid Precursor Protein-Independent Generation of Beta Amyloid in Sporadic Alzheimer's Disease
Abstract
The present review analyzes the results of recent clinical trials of β secretase inhibition in sporadic Alzheimer's disease (SAD), considers the striking dichotomy between successes in tests of β-site Amyloid Precursor Protein-Cleaving Enzyme (BACE) inhibitors in healthy subjects and familial Alzheimer's disease (FAD) models versus persistent failures of clinical trials and interprets it as a confirmation of key predictions for a mechanism of amyloid precursor protein (APP)-independent, β secretase inhibition-resistant production of β amyloid in SAD, previously proposed by us. In light of this concept, FAD and SAD should be regarded as distinctly different diseases as far as β-amyloid generation mechanisms are concerned, and whereas β secretase inhibition would be neither applicable nor effective in the treatment of SAD, the β-site APP-Cleaving Enzyme (BACE) inhibitor(s) deemed failed in SAD trials could be perfectly suitable for the treatment of FAD. Moreover, targeting the aspects of Alzheimer's disease (AD) other than cleavages of the APP by β and α secretases should have analogous impacts in both FAD and SAD.
Keywords: Alzheimer’s disease; amyloid precursor protein; amyloid precursor protein-independent generation of β amyloid; familial Alzheimer’s disease; sporadic Alzheimer’s disease; β-site amyloid precursor protein-cleaving enzyme 1 inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
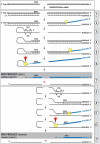
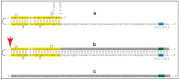
Similar articles
-
Precursor-Independent Overproduction of Beta-Amyloid in AD: Mitochondrial Dysfunction as Possible Initiator of Asymmetric RNA-Dependent βAPP mRNA Amplification. An Engine that Drives Alzheimer's Disease.Ann Integr Mol Med. 2019;1(1):61-74. Epub 2019 Nov 20. Ann Integr Mol Med. 2019. PMID: 31858090 Free PMC article.
-
Neurons derived from sporadic Alzheimer's disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation.Alzheimers Res Ther. 2017 Dec 1;9(1):90. doi: 10.1186/s13195-017-0317-z. Alzheimers Res Ther. 2017. PMID: 29191219 Free PMC article.
-
Natural Compounds with Anti-BACE1 Activity as Promising Therapeutic Drugs for Treating Alzheimer's Disease.Planta Med. 2019 Nov;85(17):1316-1325. doi: 10.1055/a-1019-9819. Epub 2019 Oct 16. Planta Med. 2019. PMID: 31618777 Review.
-
Familial Alzheimer's disease mutations inhibit gamma-secretase-mediated liberation of beta-amyloid precursor protein carboxy-terminal fragment.J Neurochem. 2005 Sep;94(5):1189-201. doi: 10.1111/j.1471-4159.2005.03266.x. Epub 2005 Jun 30. J Neurochem. 2005. PMID: 15992373
-
Evidence For and Against a Pathogenic Role of Reduced γ-Secretase Activity in Familial Alzheimer's Disease.J Alzheimers Dis. 2016 Apr 4;52(3):781-99. doi: 10.3233/JAD-151186. J Alzheimers Dis. 2016. PMID: 27060961 Review.
Cited by
-
Deep Profiling of the Aggregated Proteome in Alzheimer's Disease: From Pathology to Disease Mechanisms.Proteomes. 2018 Nov 12;6(4):46. doi: 10.3390/proteomes6040046. Proteomes. 2018. PMID: 30424485 Free PMC article. Review.
-
RNA-dependent Amplification of Mammalian mRNA Encoding Extracellullar Matrix Proteins: Identification of Chimeric RNA Intermediates for α1, β1, and γ1 Chains of Laminin.Ann Integr Mol Med. 2019;1(1):48-60. Epub 2019 Aug 25. Ann Integr Mol Med. 2019. PMID: 31663081 Free PMC article.
-
Development of an Efficient Enzyme Production and Structure-Based Discovery Platform for BACE1 Inhibitors.Biochemistry. 2019 Nov 5;58(44):4424-4435. doi: 10.1021/acs.biochem.9b00714. Epub 2019 Oct 22. Biochemistry. 2019. PMID: 31549827 Free PMC article.
-
Precursor-Independent Overproduction of Beta-Amyloid in AD: Mitochondrial Dysfunction as Possible Initiator of Asymmetric RNA-Dependent βAPP mRNA Amplification. An Engine that Drives Alzheimer's Disease.Ann Integr Mol Med. 2019;1(1):61-74. Epub 2019 Nov 20. Ann Integr Mol Med. 2019. PMID: 31858090 Free PMC article.
-
The Role of Traditional Chinese Medicine Natural Products in β-Amyloid Deposition and Tau Protein Hyperphosphorylation in Alzheimer's Disease.Drug Des Devel Ther. 2023 Nov 13;17:3295-3323. doi: 10.2147/DDDT.S380612. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 38024535 Free PMC article. Review.
References
-
- Iizuka T., Shoji M., Kawarabayashi T., Sato M., Kobayashi T., Tada N., Kasai K., Matsubara E., Watanabe M., Tomidokoro Y., et al. Intracellular generation of amyloid β-protein from amyloid β-protein precursor fragment by direct cleavage with β- and γ-secretase. Biochem. Biophys. Res. Commun. 1996;218:238–242. doi: 10.1006/bbrc.1996.0042. - DOI - PubMed
-
- DeStrooper B., Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci. 2000;113:1857–1870. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

