Elucidation of the pathology and tissue distribution of Lagovirus europaeus GI.2/RHDV2 (rabbit haemorrhagic disease virus 2) in young and adult rabbits (Oryctolagus cuniculus)
- PMID: 29866169
- PMCID: PMC5987473
- DOI: 10.1186/s13567-018-0540-z
Elucidation of the pathology and tissue distribution of Lagovirus europaeus GI.2/RHDV2 (rabbit haemorrhagic disease virus 2) in young and adult rabbits (Oryctolagus cuniculus)
Abstract
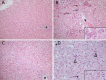
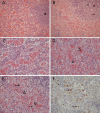
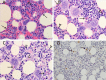
Lagovirus europaeus GI.2, also known as RHDV2 or RHDVb, is an emerging virus that causes rabbit haemorrhagic disease (RHD) in European rabbits (Oryctolagus cuniculus). In contrast to L. europaeus GI.1 (or RHDV/RHDVa) viruses that are only pathogenic for adults, GI.2 causes clinical disease in both adults and kittens. However, detailed descriptions of the pathology of this virus that may provide insight into its pathogenicity and emergence are lacking. Using an Australian GI.2 field strain isolated in 2015, we provide the first detailed description of pathology, viral antigen distribution and tissue load of GI.2 in adult and 5-week old New Zealand white rabbits using histology, immunohistochemistry and RT-qPCR. Liver was the target organ, but in contrast to GI.1 viruses, lesions and inflammatory responses did not differ between adults and kittens. Lymphocytic inflammation, proposed to be protective in kittens infected with GI.1, was notably absent. We also present the first descriptions of bone marrow changes in RHD, including decreased myeloid-to-erythroid ratio. Consistent with other pathogenic lagoviruses, intracellular viral antigen was demonstrated in hepatocytes and cells of the mononuclear phagocytic system. In terminal stages of disease, viral loads were highest in liver, serum and spleen. Despite the small sample size, our data suggest that unlike early European GI.2 strains, the pathogenicity of the Australian GI.2 virus is similar to GI.1 viruses. Additionally, GI.2 was fatal for all (n = 5) inoculated kittens in this study. This may significantly alter RHD epidemiology in the field, and may impact biocontrol programs for invasive rabbits in Australia where GI.1 viruses are intentionally released.
Figures

References
-
- Le Gall-Reculé G, Lavazza A, Marchandeau S, Bertagnoli S, Zwingelstein F, Cavadini P, Martinelli N, Lombardi G, Guerin J-L, Lemaitre E, Decors A, Boucher S, Le Normand B, Capucci L. Emergence of a new lagovirus related to rabbit haemorrhagic disease virus. Vet Res. 2013;44:81. doi: 10.1186/1297-9716-44-81. - DOI - PMC - PubMed
-
- Le Pendu J, Abrantes J, Bertagnoli S, Guitton JS, Le Gall-Reculé G, Lopes AM, Marchandeau S, Alda F, Almeida T, Célio AP, Bárcena J, Burmakina G, Blanco E, Calvete C, Cavadini P, Cooke B, Dalton K, Delibes Mateos M, Deptula W, Eden JS, Wang F, Ferreira CC, Ferreira P, Foronda P, Gonçalves D, Gavier-Widén D, Hall R, Hukowska-Szematowicz B, Kerr P, Kovaliski J, et al. Proposal for a unified classification system and nomenclature of lagoviruses. J Gen Virol. 2017;98:1658–1666. doi: 10.1099/jgv.0.000840. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

