Activated protein C, protease activated receptor 1, and neuroprotection
- PMID: 29866816
- PMCID: PMC6043978
- DOI: 10.1182/blood-2018-02-769026
Activated protein C, protease activated receptor 1, and neuroprotection
Abstract
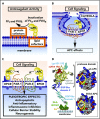
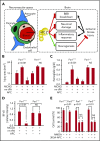
Protein C is a plasma serine protease zymogen whose active form, activated protein C (APC), exerts potent anticoagulant activity. In addition to its antithrombotic role as a plasma protease, pharmacologic APC is a pleiotropic protease that activates diverse homeostatic cell signaling pathways via multiple receptors on many cells. Engineering of APC by site-directed mutagenesis provided a signaling selective APC mutant with 3 Lys residues replaced by 3 Ala residues, 3K3A-APC, that lacks >90% anticoagulant activity but retains normal cell signaling activities. This 3K3A-APC mutant exerts multiple potent neuroprotective activities, which require the G-protein-coupled receptor, protease activated receptor 1. Potent neuroprotection in murine ischemic stroke models is linked to 3K3A-APC-induced signaling that arises due to APC's cleavage in protease activated receptor 1 at a noncanonical Arg46 site. This cleavage causes biased signaling that provides a major explanation for APC's in vivo mechanism of action for neuroprotective activities. 3K3A-APC appeared to be safe in ischemic stroke patients and reduced bleeding in the brain after tissue plasminogen activator therapy in a recent phase 2 clinical trial. Hence, it merits further clinical testing for its efficacy in ischemic stroke patients. Recent studies using human fetal neural stem and progenitor cells show that 3K3A-APC promotes neurogenesis in vitro as well as in vivo in the murine middle cerebral artery occlusion stroke model. These recent advances should encourage translational research centered on signaling selective APC's for both single-agent therapies and multiagent combination therapies for ischemic stroke and other neuropathologies.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: B.V.Z. is a founder of ZZ Biotech LLC, a biotechnology company with a mission to develop APC and its functional mutants for the treatment of stroke and other neurological disorders. L.O.M. and J.H.G. are inventors for some uses of APC mutants, and J.H.G. is a consultant for ZZ Biotech LLC.
Figures


References
-
- Seligsohn U, Lubetsky A. Genetic susceptibility to venous thrombosis. N Engl J Med. 2001;344(16):1222-1231. - PubMed
-
- Branson HE, Katz J, Marble R, Griffin JH. Inherited protein C deficiency and coumarin-responsive chronic relapsing purpura fulminans in a newborn infant. Lancet. 1983;2(8360):1165-1168. - PubMed
-
- Goldenberg NA, Manco-Johnson MJ. Protein C deficiency. Haemophilia. 2008;14(6):1214-1221. - PubMed
-
- Chalmers E, Cooper P, Forman K, et al. . Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96(11):1066-1071. - PubMed
-
- Ohga S, Ishiguro A, Takahashi Y, et al. ; Japan Childhood Thrombophilia Study Group. Protein C deficiency as the major cause of thrombophilias in childhood. Pediatr Int. 2013;55(3):267-271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

