Transdifferentiation of human adult peripheral blood T cells into neurons
- PMID: 29866841
- PMCID: PMC6016798
- DOI: 10.1073/pnas.1720273115
Transdifferentiation of human adult peripheral blood T cells into neurons
Abstract
Human cell models for disease based on induced pluripotent stem (iPS) cells have proven to be powerful new assets for investigating disease mechanisms. New insights have been obtained studying single mutations using isogenic controls generated by gene targeting. Modeling complex, multigenetic traits using patient-derived iPS cells is much more challenging due to line-to-line variability and technical limitations of scaling to dozens or more patients. Induced neuronal (iN) cells reprogrammed directly from dermal fibroblasts or urinary epithelia could be obtained from many donors, but such donor cells are heterogeneous, show interindividual variability, and must be extensively expanded, which can introduce random mutations. Moreover, derivation of dermal fibroblasts requires invasive biopsies. Here we show that human adult peripheral blood mononuclear cells, as well as defined purified T lymphocytes, can be directly converted into fully functional iN cells, demonstrating that terminally differentiated human cells can be efficiently transdifferentiated into a distantly related lineage. T cell-derived iN cells, generated by nonintegrating gene delivery, showed stereotypical neuronal morphologies and expressed multiple pan-neuronal markers, fired action potentials, and were able to form functional synapses. These cells were stable in the absence of exogenous reprogramming factors. Small molecule addition and optimized culture systems have yielded conversion efficiencies of up to 6.2%, resulting in the generation of >50,000 iN cells from 1 mL of peripheral blood in a single step without the need for initial expansion. Thus, our method allows the generation of sufficient neurons for experimental interrogation from a defined, homogeneous, and readily accessible donor cell population.
Keywords: direct conversion; disease modeling; iN cells; induced neuronal cells; transdifferentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
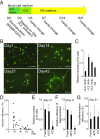
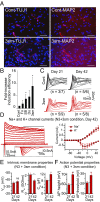
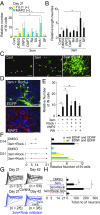


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

