Water agglomerates on Fe3O4(001)
- PMID: 29866854
- PMCID: PMC6016784
- DOI: 10.1073/pnas.1801661115
Water agglomerates on Fe3O4(001)
Abstract
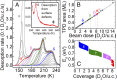
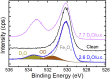
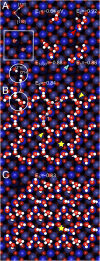
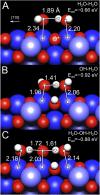
Determining the structure of water adsorbed on solid surfaces is a notoriously difficult task and pushes the limits of experimental and theoretical techniques. Here, we follow the evolution of water agglomerates on Fe3O4(001); a complex mineral surface relevant in both modern technology and the natural environment. Strong OH-H2O bonds drive the formation of partially dissociated water dimers at low coverage, but a surface reconstruction restricts the density of such species to one per unit cell. The dimers act as an anchor for further water molecules as the coverage increases, leading first to partially dissociated water trimers, and then to a ring-like, hydrogen-bonded network that covers the entire surface. Unraveling this complexity requires the concerted application of several state-of-the-art methods. Quantitative temperature-programmed desorption (TPD) reveals the coverage of stable structures, monochromatic X-ray photoelectron spectroscopy (XPS) shows the extent of partial dissociation, and noncontact atomic force microscopy (AFM) using a CO-functionalized tip provides a direct view of the agglomerate structure. Together, these data provide a stringent test of the minimum-energy configurations determined via a van der Waals density functional theory (DFT)-based genetic search.
Keywords: Fe3O4; H-bond network; cooperativity; magnetite; water.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Björneholm O, et al. Water at interfaces. Chem Rev. 2016;116:7698–7726. - PubMed
-
- Hodgson A, Haq S. Water adsorption and the wetting of metal surfaces. Surf Sci Rep. 2009;64:381–451.
-
- Maier S, Salmeron M. How does water wet a surface? Acc Chem Res. 2015;48:2783–2790. - PubMed
-
- Henderson MA. The interaction of water with solid surfaces: Fundamental aspects revisited. Surf Sci Rep. 2002;46:1–308.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

