Nucleotide Prodrug Containing a Nonproteinogenic Amino Acid To Improve Oral Delivery of a Hepatitis C Virus Treatment
- PMID: 29866875
- PMCID: PMC6105845
- DOI: 10.1128/AAC.00620-18
Nucleotide Prodrug Containing a Nonproteinogenic Amino Acid To Improve Oral Delivery of a Hepatitis C Virus Treatment
Abstract
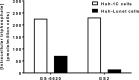
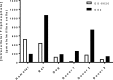
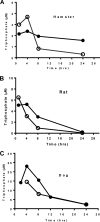
Delivery of pharmacologically active nucleoside triphosphate analogs to sites of viral infection is challenging. In prior work we identified a 2'-C-methyl-1'-cyano-7-deaza-adenosine C-nucleotide analog with desirable selectivity and potency for the treatment of hepatitis C virus (HCV) infection. However, the prodrug selected for clinical development, GS-6620, required a high dose for meaningful efficacy and had unacceptable variability due to poor oral absorption as a result of suboptimal solubility, intestinal metabolism, and efflux transport. While obtaining clinical proof of concept for the nucleotide analog, a more effective prodrug strategy would be necessary for clinical utility. Here, we report an alternative prodrug of the same nucleoside analog identified to address liabilities of GS-6620. A phosphoramidate prodrug containing the nonproteinogenic amino acid methylalanine, an isopropyl ester and phenol in the (S) conformation at phosphorous, GS2, was found to have improved solubility, intestinal stability, and hepatic activation. GS2 is a more selective substrate for hepatically expressed carboxyl esterase 1 (CES1) and is resistant to hydrolysis by more widely expressed hydrolases, including cathepsin A (CatA) and CES2. Unlike GS-6620, GS2 was not cleaved by intestinally expressed CES2 and, as a result, was stable in intestinal extracts. Levels of liver triphosphate following oral administration of GS2 in animals were higher than those of GS-6620, even when administered under optimal conditions for GS-6620 absorption. Combined, these properties suggest that GS2 will have better oral absorption in the clinic when administered in a solid dosage form and the potential to extend the clinical proof of concept obtained with GS-6620.
Keywords: GS-6620; GS2; HCV; antiviral; antiviral agents; carboxyl esterase; carboxyl esterase 1; cathepsin A; nucleotide prodrug.
Copyright © 2018 Feng et al.
Figures



References
-
- Watkins WJ, Ray AS, Chong LS. 2010. HCV NS5B polymerase inhibitors. Curr Opin Drug Discov Dev 13:441–465. - PubMed
-
- Arnold JJ, Sharma SD, Feng JY, Ray AS, Smidansky ED, Kireeva ML, Cho A, Perry J, Vela JE, Park Y, Xu Y, Tian Y, Babusis D, Barauskus O, Peterson BR, Gnatt A, Kashlev M, Zhong W, Cameron CE. 2012. Sensitivity of mitochondrial transcription and resistance of RNA polymerase II dependent nuclear transcription to antiviral ribonucleosides. PLoS Pathog 8:e1003030. doi:10.1371/journal.ppat.1003030. - DOI - PMC - PubMed
-
- Feng JY, Xu Y, Barauskas O, Perry JK, Ahmadyar S, Stepan G, Yu H, Babusis D, Park Y, McCutcheon K, Perron M, Schultz BE, Sakowicz R, Ray AS. 2016. Role of mitochondrial RNA polymerase in the toxicity of nucleotide inhibitors of hepatitis C virus. Antimicrob Agents Chemother 60:806–817. doi:10.1128/AAC.01922-15. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

