Ethanolamine ameliorates mitochondrial dysfunction in cardiolipin-deficient yeast cells
- PMID: 29866881
- PMCID: PMC6052229
- DOI: 10.1074/jbc.RA118.004014
Ethanolamine ameliorates mitochondrial dysfunction in cardiolipin-deficient yeast cells
Abstract
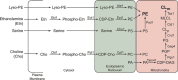
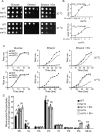
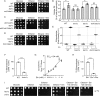
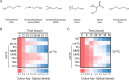
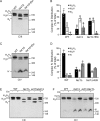
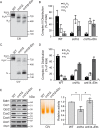
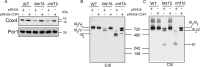
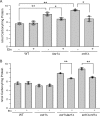
Cardiolipin (CL) is a signature phospholipid of the mitochondria required for the formation of mitochondrial respiratory chain (MRC) supercomplexes. The destabilization of MRC supercomplexes is the proximal cause of the pathology associated with the depletion of CL in patients with Barth syndrome. Thus, promoting supercomplex formation could ameliorate mitochondrial dysfunction associated with CL depletion. However, to date, physiologically relevant small-molecule regulators of supercomplex formation have not been identified. Here, we report that ethanolamine (Etn) supplementation rescues the MRC defects by promoting supercomplex assembly in a yeast model of Barth syndrome. We discovered this novel role of Etn while testing the hypothesis that elevating mitochondrial phosphatidylethanolamine (PE), a phospholipid suggested to overlap in function with CL, could compensate for CL deficiency. We found that the Etn supplementation rescues the respiratory growth of CL-deficient Saccharomyces cerevisiae cells in a dose-dependent manner but independently of its incorporation into PE. The rescue was specifically dependent on Etn but not choline or serine, the other phospholipid precursors. Etn improved mitochondrial function by restoring the expression of MRC proteins and promoting supercomplex assembly in CL-deficient cells. Consistent with this mechanism, overexpression of Cox4, the MRC complex IV subunit, was sufficient to promote supercomplex formation in CL-deficient cells. Taken together, our work identifies a novel role of a ubiquitous metabolite, Etn, in attenuating mitochondrial dysfunction caused by CL deficiency.
Keywords: Barth syndrome; cardiolipin; cytochrome c oxidase (Complex IV); ethanolamine; mitochondria; mitochondrial respiratory chain complex; phospholipid; respiratory supercomplexes.
© 2018 Basu Ball et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

