Novel Role of VisP and the Wzz System during O-Antigen Assembly in Salmonella enterica Serovar Typhimurium Pathogenesis
- PMID: 29866904
- PMCID: PMC6056878
- DOI: 10.1128/IAI.00319-18
Novel Role of VisP and the Wzz System during O-Antigen Assembly in Salmonella enterica Serovar Typhimurium Pathogenesis
Abstract
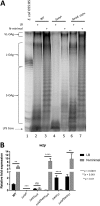
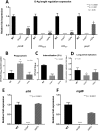
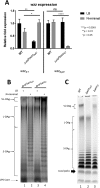
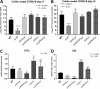
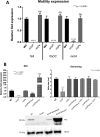
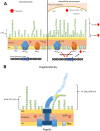
Salmonella enterica serovars are associated with diarrhea and gastroenteritis and are a helpful model for understanding host-pathogen mechanisms. Salmonella enterica serovar Typhimurium regulates the distribution of O antigen (OAg) and presents a trimodal distribution based on Wzy polymerase and the WzzST (long-chain-length OAg [L-OAg]) and WzzfepE (very-long-chain-length OAg [VL-OAg]) copolymerases; however, several mechanisms regulating this process remain unclear. Here, we report that LPS modifications modulate the infectious process and that OAg chain length determination plays an essential role during infection. An increase in VL-OAg is dependent on Wzy polymerase, which is promoted by a growth condition resembling the environment of Salmonella-containing vacuoles (SCVs). The virulence- and stress-related periplasmic protein (VisP) participates in OAg synthesis, as a ΔvisP mutant presents a semirough OAg phenotype. The ΔvisP mutant has greatly decreased motility and J774 macrophage survival in a colitis model of infection. Interestingly, the phenotype is restored after mutation of the wzzST or wzzfepE gene in a ΔvisP background. Loss of both the visP and wzzST genes promotes an imbalance in flagellin secretion. L-OAg may function as a shield against host immune systems in the beginning of an infectious process, and VL-OAg protects bacteria during SCV maturation and facilitates intramacrophage replication. Taken together, these data highlight the roles of OAg length in generating phenotypes during S Typhimurium pathogenesis and show the periplasmic protein VisP as a novel protein in the OAg biosynthesis pathway.
Keywords: LPS; O antigen; Salmonella; VisP; lipopolysaccharide.
Copyright © 2018 American Society for Microbiology.
Figures
Similar articles
-
Regulation of Salmonella typhimurium lipopolysaccharide O antigen chain length is required for virulence; identification of FepE as a second Wzz.Mol Microbiol. 2003 Mar;47(5):1395-406. doi: 10.1046/j.1365-2958.2003.03383.x. Mol Microbiol. 2003. PMID: 12603743
-
Salmonella Typhimurium O-antigen and VisP play an important role in swarming and osmotic stress response during intracellular conditions.Braz J Microbiol. 2022 Jun;53(2):557-564. doi: 10.1007/s42770-022-00701-9. Epub 2022 Mar 18. Braz J Microbiol. 2022. PMID: 35303296 Free PMC article.
-
Genetic modulation of Shigella flexneri 2a lipopolysaccharide O antigen modal chain length reveals that it has been optimized for virulence.Microbiology (Reading). 2003 Apr;149(Pt 4):925-939. doi: 10.1099/mic.0.26141-0. Microbiology (Reading). 2003. PMID: 12686635
-
Autophagy recognizes intracellular Salmonella enterica serovar Typhimurium in damaged vacuoles.Autophagy. 2006 Jul-Sep;2(3):156-8. doi: 10.4161/auto.2825. Epub 2006 Jul 10. Autophagy. 2006. PMID: 16874057 Review.
-
Recent insights into Wzy polymerases and lipopolysaccharide O-antigen biosynthesis.J Bacteriol. 2025 Apr 17;207(4):e0041724. doi: 10.1128/jb.00417-24. Epub 2025 Mar 11. J Bacteriol. 2025. PMID: 40066993 Free PMC article. Review.
Cited by
-
Mutation in Wzz(fepE) Linked to Altered O-Antigen Biosynthesis and Attenuated Virulence in Rough Salmonella Infantis Variant.Vet Sci. 2024 Nov 28;11(12):603. doi: 10.3390/vetsci11120603. Vet Sci. 2024. PMID: 39728943 Free PMC article.
-
Genome-wide Analysis of Salmonella enterica serovar Typhi in Humanized Mice Reveals Key Virulence Features.Cell Host Microbe. 2019 Sep 11;26(3):426-434.e6. doi: 10.1016/j.chom.2019.08.001. Epub 2019 Aug 22. Cell Host Microbe. 2019. PMID: 31447308 Free PMC article.
-
PgtE protease enables virulent Salmonella to evade C3-mediated serum and neutrophil killing.bioRxiv [Preprint]. 2024 Nov 5:2024.11.05.622138. doi: 10.1101/2024.11.05.622138. bioRxiv. 2024. Update in: mBio. 2025 Aug 13;16(8):e0380224. doi: 10.1128/mbio.03802-24. PMID: 39574608 Free PMC article. Updated. Preprint.
-
Chemotaxis and Shorter O-Antigen Chain Length Contribute to the Strong Desiccation Tolerance of a Food-Isolated Cronobacter sakazakii Strain.Front Microbiol. 2022 Jan 4;12:779538. doi: 10.3389/fmicb.2021.779538. eCollection 2021. Front Microbiol. 2022. PMID: 35058898 Free PMC article.
-
QseC Signaling in the Outbreak O104:H4 Escherichia coli Strain Combines Multiple Factors during Infection.J Bacteriol. 2019 Aug 8;201(17):e00203-19. doi: 10.1128/JB.00203-19. Print 2019 Sep 1. J Bacteriol. 2019. PMID: 31235511 Free PMC article.
References
-
- World Health Organization. 2013. Salmonella (non-typhoidal), fact sheet no. 139. World Health Organization, Geneva, Switzerland.
-
- Feasey NA, Cain AK, Msefula CL, Pickard D, Alaerts M, Aslett M, Everett DB, Allain TJ, Dougan G, Gordon MA, Heyderman RS, Kingsley RA. 2014. Drug resistance in Salmonella enterica ser. Typhimurium bloodstream infection, Malawi. Emerg Infect Dis 20:1957–1959. doi:10.3201/eid2011.141175. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

